[0001] 20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT
[0002]IMIDAZO[5,1-C][1,2,4]BENZOTRIAZINE DERIVATIVES AS INHIBITORS OF PHOSPHODIESTERASES
[0003]TECHNICAL FIELD The invention relates to imidazo[5,l-c][l,2,4]benzotnazine deπvatives which are inhibitors of phosphodiesterase 2 or 10, useful in treating central nervous system diseases such as psychosis and also in treating, for example, obesity, type 2 diabetes, metabolic syndrome, glucose intolerance, and pain
[0004]BACKGROUND Psychotic disorders, especially schizophrenia, are severe mental disorders which extremely impair daily life The symptoms of psychosis may be divided into two fractions In the acute phase, it is predominated by hallucinations and delusions being called the positive symptoms When the agitated phase abates the so called negative symptoms become obvious They include cognitive deficits, social phobia, reduced vigilance, indifference and deficits in verbal learning and memory, verbal fluency and motor function
[0005]Although several antipsychotics have become available, the present therapy of psychosis is not satisfactory The classic antipsychotics, such as halopendol, with a high affinity to dopamine D2 receptor show extreme side effects, such as extrapyramidal symptoms (=EPS) and do not improve the negative symptoms of schizophrenia so that they do not enable the patient to return to everyday life Other antipsychotics, such as clozapine, can show negative side effects, such as agranulocytosis
[0006]In addition to psychotic disorders, depression is a severe mental disorder which extremely impairs daily life Its prevalence is about 10 % of the world population with an incidence of 2 % according to WHO Women are more affected than men and elder people more than younger people The disorder mostly implies a life-long treatment due to the progress of the disease and permanent total disability
[0007]The most prominent symptoms of the disease are anhedoma, feeling of hopelessness, decreased self esteem, loss of appetite and sleep disturbance Most patients
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT are suicidal Depression is often combined with anxiety disorders Interestingly, it is less known that depression is also regularly associated with various cognitive impairments (Gualtien et al , 2006, Mandelli et al , 2006) Here, deficits of attentional and executive function are mostly reported (Paelecke-Habermann et a , 2005) Cognitive deficits are even discussed to be involved in the development of the disease (Beck depression model, Beck, 2008) Actually, the seventy of the cognitive deficits may predict non-response to certain antidepressant treatment (Dunkin et al , 2000, Gorlyn et al , 2008)
[0008]Elder antidepressants are reported to impair memory in animal models of learning and memory probably due to their anticholinergic component (Kumar and Kulkarm, 1996) In contrast, SSRIs, especially fluoxetine, are descπbed to impair hippocampal- independent but not hippocampal dependent learning in different rodent models (Valluzi and Chan, 2007) Some modern antidepressants are described to reverse cognitive impairments associated with stress-induced depression in rats (Ramanathan et al , 2003) At least, m clinic current therapy it is not possible to fully reverse cognitive deficits Thus, in depressive patients who had been successfully treated cognitive performance could be improved but not normalised (Gualtien et al , 2006) Therefore, an antidepressant with higher efficacy on cognitive impairment may improve disease outcome
[0009]Phosphodiesterases (PDE) are expressed m nearly all mammalian cells As a consequence, they play an important role in numerous physiological and pathophysiological processes To date eleven families of phosphodiesterases have been identified in mammals (Essayan, 2001) It is well established that PDEs are cntically involved in cell signalling Specifically, PDEs are known to inactivate the cyclic nucleotides cAMP and/or cGMP (Soderlmg and Beavo, 2000) The cyclic nucleotides cAMP and cGMP are synthesised by the adenylyl and guanylyl cyclases and are second messengers that control many key cellular functions The synthesis of cAMP and cGMP is regulated by different G-protein-coupled receptor types including dopamine Dl and D2 receptors By its effect PDEs may reduce or even eliminate the signal cascade initiated by activating extracellular receptors PDE inhibitors, in contrast, may prolong or amplify this effect Thereby the different phosphodiesterase families and their inhibitors may very
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT specifically participate in the maintenance and the regulation of the homeostasis of an organism
[0010]The phosphodiesterases of the different families vary m their substrate selectivity
[0011]Thus, some families only hydrolyse cAMP others only cGMP Some phosphodiesterases inactivate both cAMP and cGMP (Menniti et al , 2006) Furthermore, there is a difference in the distribution of the different phosphodiesterases withm the organism and additionally, withm any particular tissue or organ For instance, the distribution pattern of the phosphodiesterases within the brain is quite specific (Menniti et al , 2006)
[0012]Finally, phosphodiesterase families have different regulatory properties and intracellular location, some are bound to cell membranes and some are dissociated in the cytoplasm, additionally, a division into various intracellular compartments has been reported (Conti and Jin, 1999)
[0013]These differences in the function and location of the different PDE enzyme families suggests that the individual phosphodiesterases are selectively involved in regulating many different physiological processes Accordingly, selective phosphodiesterase inhibitors may with fine specificity regulate different physiological and pathophysiological processes
[0014]PDE2 hydrolyses both, cGMP and cAMP and is activated by cGMP (Menniti et al , 2006) It is abundantly expressed in the brain (Bolger et al , 1994) Here, PDE2 mRNA is mainly distributed in olfactory bulb, olfactory tubercle, cortex, amygdala, striatum, and hippocampus (Lakics et al , 2005, van Staveren et al , 2003)
[0015]The expression of PDE2 m the hippocampus and the cortex indicate an involvement m the mechanism of learning and memory This is supported by the fact that increased levels of both cGMP and cAMP are involved in the process of LTP forming (Blokland et al , 2006, Pπckaerts et al , 2002) LTP is regarded as the electrophysiological basis of long term memory (Baddeley, 2003) Boess et al (2004) showed that PDE2 inhibitors amplify the generation of long term potentiation (LTP)
[0016]Additionally, it is reported that the selective PDE2 inhibitor BAY60-7550 enhances learning and memory in rats and mice in different animal models (Boess et al , 2004, Rutten et al , 2006) Thus, BAY60-7550 is efficacious in the novel object recognition test, the social recognition test and the T-maze, an animal model of working memory
20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
[0017]Furthermore, the expression of PDE2 m the nucleus accumbens (part of the striatum), the olfactory bulb, the olfactory tubercle and the amygdale supports additional involvement of PDE2 in the pathophysiology of anxiety and depression (Modell et al , 1990) As described above, PDE2 inhibitors increase cAMP and cGMP in neuronal cells There is evidence that chrome administration of antidepressants up-regulates the cAMP pathway at several levels, including increased expression of the cAMP response element binding protein (CREB) (Duman, 1998, Nibuya et al , 1996) In contrast, patients with depression show an impairment of the cAMP pathway Thus, Shelton et al (1999) detected a reduction of cAMP associated protein kinase A in depressive patients Finally, Masood et al (2008) report an anxiolytic effect of PDE2 inhibition m mice They reversed oxidative stress-induced anxiety by the PDE2 inhibitor BAY60-7550
[0018]Consequently, PDE2 inhibitors are descπbed to have a potential to alleviate central nervous system (CNS) disorders, e g depression and Alzheimer's disease but also peripheral diseases like metabolic disorders, septic shock and cancer PDElO (PDElOA) is primarily expressed in the bram and here in the nucleus accumbens and the caudate putamen Areas with moderate expression are the thalamus, hippocampus, frontal cortex and olfactory tubercle (Menniti et al , William Harvey Research Conference, Porto, December 6th - 8th, 2001) All these bram areas are descπbed to participate in the pathomechamsm of schizophrenia (Lapiz et al , Neurosci Behav Physiol 33 13-29, 2003) so that the location of the enzyme indicates a predominate role in the pathomechamsm of psychosis
[0019]PDE2 inhibitors address a novel target in the bram PDE2 inhibitors are descπbed to have an antidepressant and anxiolytic effect Additionally, they improve impaired but also un-impaired learning and memory (Boess et al , 2004, Rutten et al , 2006b) Thus, PDE2 inhibitors are a promising new target to improve the therapy of CNS disorders, especially depression and Alzheimer's disease
[0020]Several families of PDE2 inhibitors are known Imidazotπazinones are claimed in WO 02068423 for the treatment of e g memory deficiency, cognitive disorders, dementia and Alzheimer's disease Oxmdoles are descnbed in WO 05041957 for the treatment of dementia Further inhibitors of PDE2 are known from WO 07121319 for the treatment of anxiety and depression, from WO 06072615, WO 06072612, WO 06024640 and WO
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT
[0021]05113517 for the treatment of arthritis, cancer, edema and septic shock, from WO 05063723 for the treatment of renal and liver failure, liver dysfunction, restless leg syndrom, rheumatic disorders, arthritis, rhinitis, asthma and obesity, from WO 05041957 for the treatment of cancer and thrombotic disorders, from WO 06102728 for the treatment of angina pectoris and hypertension from WO 08043461 for the treatment of cardiovascular disorders, erectile dysfunction, inflammation and renal failure and from WO 05061497 for the treatment of e g dementia, memory disorders, cancer and osteoporosis
[0022]Finally, benzodiazepines are claimed in WO 2005063723 for the general treatment of CNS diseases including anxiety, depression, ADHD, neurodegeneration, Alzheimer's disease and psychosis
[0023]Unfortunately, there is no PDE2 inhibitor that could be successfully developed to become a treatment medication Most of them are not optimal for CNS penetration or suffer on pure physical properties There is still an urgent need to provide new PDE2 inhibitors with improved properties for the treatment of diseases where inhibition of PDE2 is of therapeutic value
[0024]In the stratum PDElOA is predominately found in the medium spmy neurons and they are primarily associated to the postsynaptic membranes of these neurons (Xie et al , Neuroscience 139 597-607, 2006) By this location PDElOA may have an important influence on the signal cascade induced by dopaminergic and glutamatergic input on the medium spiny neurons two neurotransmitter systems playing a predominate role in the pathomechamsm of psychosis
[0025]Phosphodiesterase (PDE) 1OA, in particular, hydrolyses both cAMP and cGMP having a higher affinity for cAMP (Km = 0 05 μM) than for cGMP (Km =3 μM) (Soderlmg et al , Curr Opin Cell Biol 12 174-179, 1999)
[0026]Psychotic patients have been shown to have a dysfunction of cGMP and cAMP levels and its downstream substrates (Kaiya, Prostaglandins Leukot Essent Fatty Acids 46 33-38, 1992, MuIy, Psychopharmacol Bull 36 92-105, 2002, Garver et al , Life Sci 31 1987- 1992, 1982) Additionally, halopeπdol treatment has been associated with increased cAMP and cGMP levels m rats and patients, respectively (Leveque et al , J Neurosci 20 4011-4020, 2000, Gattaz et al , Biol Psychiatry 19 1229-1235, 1984) As
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0027]PDElOA hydrolyses both cAMP and cGMP (Kotera et al , Biochem Biophys Res Commun 261 551-557, 1999), an inhibition of PDElOA would also induce an increase of cAMP and cGMP and thereby have a similar effect on cyclic nucleotide levels as halopendol The antipsychotic potential of PDElOA inhibitors is further supported by studies of Kostowski et al (Pharmacol Biochem Behav 5 15-17, 1976) who showed that papaveπne, a moderate selective PDElOA inhibitor, reduces apomorphme-mduced stereotypies in rats, an animal model of psychosis, and increases halopeπdol-induced catalepsy in rats while concurrently reducing dopamine concentration in rat brain, activities that are also seen with classical antipsychotics This is further supported by a patent application establishing papaveπne as a PDElOA inhibitor for the treatment of psychosis (US Patent Application Pub No 2003/0032579)
[0028]In addition to classical antipsychotics which mamly ameliorate the positive symptoms of psychosis, PDElOA also bears the potential to improve the negative and cognitive symptoms of psychosis
[0029]Focusing on the dopaminergic input on the medium spiny neurons, PDElOA inhibitors by up-regulatmg cAMP and cGMP levels act as Dl agonists and D2 antagonists because the activation of Gs-protem coupled dopamine Dl receptor increases intracellular cAMP, whereas the activation of the Gi-protem coupled dopamine D2 receptor decreases intracellular cAMP levels through inhibition of adenylyl cyclase activity (Mutschler et al , Mutschler Arzneimittelwirkungen 8th ed Stuttgart Wissenschaftliche Verlagsgesellschaft mbH, 2001)
[0030]Elevated intracellular cAMP levels mediated by Dl receptor signalling seems to modulate a series of neuronal processes responsible for working memory in the prefrontal cortex (Sawaguchi, Parkinsonism Relat Disord 7 9-19, 2000), and it is reported that Dl receptor activation may improve working memory deficits in schizophrenic patients (Castner et al , Science 287 2020-2022, 2000)
[0031]Further indication of an effect of PDElOA inhibition on negative symptoms of psychosis was given by Rodefer et al (Eur J Neurosci 21 1070-1076, 2005) who could show that papaveπne reverses attentional set-shiftmg deficits induced by subchromc administration of phencyclidine, an NMDA antagonist, in rats Attentional deficits
20743-0032WOi (AM103539, 44418, HUBR-1344 PCT) PATENT including an impairment of shifting attention to novel stimuli belongs to the negative symptoms of schizophrenia In the study the attentional deficits were induced by administering phencychdme for 7 days followed by a washout period The PDElOA inhibitor papaverine was able to reverse the enduπng deficits induced by the subchromc treatment
[0032]Several routes are descπbed for the synthesis of 1 ,2,4-Benzotπazines Sadchikova et al published an intermolecular cyclisation of 2-hydroxyphenyl- or 2- methoxyphenylazo dyes (Sadchikova et al 2000 and 2005) An other opportunity is the reaction of l-(2-aminophenyl)-irmdazoles with sodium nitrite and H2SO4 In this case the resulting tπazme was observed as a side product in low yields (6%) only (Antomm et al 1976, Simonov et al 1969 and 1967) In US 2006/0111568 a number of 1,2,4- benzotnazines are claimed to be azo dyes These derivatives are substituted with cyano groups at the imidazole ring and with hydroxyl groups at the benzo πng of the molecule Salts of similar derivatives were claimed in DE 2348382 In this case the benzo πng needed to be substituted with an ammo group In WO 2005/014595 the use of benzol ,2,4-tπazines as a herbicide or plant growth regulator is claimed
[0033]In conclusion, there is a need for new antipsychotic and antidepressant agents This invention addresses this need and others
[0035]The present invention provides, inter aha, compounds of formula (I)
[0036]
[0037]I or pharmaceutically acceptable salts thereof
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0038]The present invention also provides a pharmaceutical composition comprising a compound of formula (I), or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable earner
[0039]The present invention further provides a method of treating disorders associated with phosphodiesterase 2 or 10 hyperactivity, the method comprising administering to a patient in need thereof a therapeutically effective amount of a compound of formula (I), or a pharmaceutically acceptable salt thereof
[0040]The present invention also provides a method of treating a central nervous system disorder in a patient m need thereof comprising, admimsteπng to said patient a therapeutically effective amount of a compound of formula (I), or a pharmaceutically acceptable salt thereof
[0041]The present invention further provides a method of treating obesity, type II diabetes, metabolic syndrome, glucose intolerance and related health risks, symptoms or disorders in a patient in need thereof composing administering to said patient a therapeutically effective amount of a compound of formula (I), or a pharmaceutically acceptable salt thereof
[0042]The present invention also provides a method of treating or preventing disorders associated with enhanced endothelial activity, impaired endothelial barrier or enhanced neoangiogenesis, septic shock, vascular edema, reduced natπuπa pathology, inflammatory diseases, asthma, rhinitis, arthritis, rheumatoid diseases, autoimmune diseases, acute renal or liver failure, liver dysfunction, and benign or malignant neoplasia in a patient m need thereof comprising, administering to said patient a therapeutically effective amount of a compound of formula (I), or a pharmaceutically acceptable salt thereof The present invention further provides a method of treating or preventing a disorder associated with thrombosis or embolism in a patient in need thereof comprising, administering to said patient a therapeutically effective amount of a compound of formula (I), or a pharmaceutically acceptable salt thereof
[0043]The present invention still further provides a method of treating pain or a pain disorder selected from inflammatory pain, hyperalgesia, inflammatory hyperalgesia, migraine, cancer pain, osteoarthritis pain, post-surgical pain, non-inflammatory pain,
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT neuropathic pain, sub-categories of neuropathic pam including peripheral neuropathic pain syndromes, chemotherapy-mduced neuropathy, complex regional pam syndrome, HIV sensory neuropathy, neuropathy secondary to tumor infiltration, painful diabetic neuropathy, phantom limb pain, postherpetic neuralgia, postmastectomy pain, trigeminal neuralgia, central neuropathic pain syndromes, central poststroke pam, multiple sclerosis pam, Parkinson disease pam, and spinal cord injury pain in a patient in need thereof, comprising admimstenng to said patient a compound of formula (I), or a pharmaceutically acceptable salt thereof
[0044]The present invention also provides a compound for use m any of the methods descπbed herein The present invention further provides use of a compound for the preparation of a medicament for use in any of the methods descnbed herein
[0045]The details of one or more embodiments of the invention are set forth m the accompanying drawings and the descnption below Other features, objects, and advantages of the invention will be apparent from the descnption and drawings, and from the claims
[0046]DETAILED DESCRIPTION
[0047]The present invention provides, inter aha, a compound of formula (I)
[0048]
[0049]I or a pharmaceutically acceptable salt thereof, wherein a, b, c, and d indicate four possible positions on the πng for each R3, when present, p is 0 or an integer from 1 to 4,
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0050]R1 is selected from hydrogen, R4, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, - C(O)R4, -C(O)OR4, -0-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -Y1- NR5R6, -Y'-N(R7) -Y2-NR8R9, -Y'-NfR'^-Y^R4, and -P(O)(OR4)2, wherein q is 1 or 2,
[0051]R2 is selected from hydrogen, R4, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, - C(O)R4, -C(O)OR4, -0-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -Y1- NR5R6, -Y'-N(R7) -Y2-NR8R9, -Y'-N^'^-Y^R4, and -P(O)(OR4)2, wherein q is 1 or 2, each R3 is independently selected from R4, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, -C(O)R4, -C(O)OR4, -0-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -Y'-NR5R6, -Y'-N(R7) -Y2-NRSR9, -Y'-NfR'^-Y^R4, and -P(O)(OR4)2, wherein q is 1 or 2, any two groups R3 may together be alkylene or alkenylene completing a 3- to 8- membered saturated or unsaturated πng together with the carbon atoms to which they are attached, which πng is unsubstituted or substituted with one or more independently selected Z groups, or any two groups of R3 may, together with the atoms to which they are attached, form a heterocyclo group which is unsubstituted or substituted with one or more independently selected Z groups, each R4 is independently selected from alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, cycloalkenylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl, each of which is unsubstituted or substituted with one or more independently selected Z groups, each R5, R6, R7, R8, R9 and R10 is independently selected from hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, cycloalkenylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl, each of which is unsubstituted or substituted with one or more independently selected Z groups, or
[0052]R5 and R6 may together be alkylene or alkenylene, completing a 3- to 8- membered saturated or unsaturated πng with the nitrogen atom to which they are attached, which nng is unsubstituted or substituted with one or more independently selected Z groups, or any two of R7, R8 and R9 may together be alkylene or alkenylene, completing a 3- to 8-membered saturated or unsaturated πng with the nitrogen atom to which they are
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT attached, which nng is unsubstituted or substituted with one or more independently selected Z groups, each Z group is independently selected from hydrogen, R11, -OH, -OR11, -SH, -SR11, -C(O)H, -C(O)OH, -C(O)R11, -C(O)OR11, -0-C(O)R11, -0-C(O)OR11, -SO3H, -S(O)qRn, halo, cyano, mtro, -Y^NR12R13, -Y'-N(R14)-Y2-NR15R16, -Y1-N(RI7)-Y2-R11, and oxo, wherein q is 1 or 2, each R11 is independently selected from alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, cycloalkenylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl, each of which is unsubstituted or substituted with one or more Z1 groups, each R12, R13, R14, Rls, R16, and R17 is independently selected from hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, cycloalkenylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl, each of which is unsubstituted or substituted with one or more independently selected Z1 groups, each Y1 and Y2 is independently selected from a single bond, -Y3-S(O)q-Y4-, -Y3-C(O)-Y4-, -Y3-C(S)-Y4-, -Y^O-Y4-, -Y3-S-Y4-, -Y^O-C(O)-Y4-, and -Y3-C(O)-O-
[0053]Y4-, each Y3 and Y4 is independently selected from a single bond, alkylene, alkenylene, and alkynylene, and each Z1 is independently selected from oxo, halogen, cyano, mtro, hydroxyl, Ci β alkyl, Ci βhaloalkyl, Ci β alkoxy, Ci6 haloalkoxy, Ci6 alkylthio, Ci β alkylsulfmyl, Ci e alkylsulfonyl, amino, Ci6 alkylammo, di-Ci6-alkylarmno, Ci β alkylcarbonyl, Ci β alkoxycarbonyl, carboxy, carbamyl, Ci6 alkylcarbamyl, di-Ci β alkylcarbamyl, Ci6 alkylcarbamyloxy, and di-Ci6-alkylcarbamyloxy hi some embodiments, the following provisos apply
[0054](a) when p is O, then R1 and R2 are not each H or each methyl,
[0055](b) when R1 is H, R2 is -C(O)O-(ethyl), p is 1 , and R3 is at the c position of the nng, then R3 is other than hydroxyl,
[0056](c) when R1 is H, R2 is mtro or -C(O)O-(ethyl), p is 2, and each R3 is at the a and c positions of the nng, then each R3 is other than methoxy,
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0057](d) when R1 is H, p is 2, each R3 is methyl, and the two R3 groups are at the a and c positions of the πng, then R2 is other than -C(O)OH, -C(O)O-(ethyl), or benzylthio,
[0058](e) when R1 is H, R2 is mtro, -C(O)O-(ethyl), -C(O)NH2, or -CONH- (methyl), p is 1 , and R3 is at the b position of the πng, then R3 is other than methyl,
[0059](f) when R1 is H, R2 is -C(O)O-(ethyl), p is 1 , and R3 is at the b position of the πng, then R3 is other than amino,
[0060](g) when R1 is methyl, l,3-dixolan-2-yl, hydroxymethyl, or formyl, and R2 is H, then p is other than 0,
[0061](h) when R1 is H, R2 is H, p is 1 , and R3 is at the c position of the πng, then R3 is other than chloro or dimethylammo,
[0062](i) when R1 is H, p is 1 , R3 is hydroxyl, and R3 is at the c position of the nng, then R2 other than cyano,
[0063](]) when R2 is H, p is 1, R3 is hydroxyl, and R3 is at the c position of the nng, then R1 other than cyano,
[0064](k) when R1 is H, R2 is H, p is 1 , and R3 is at the b position of the πng, then R3 is other than chloro, bromo, methyl, methoxy, mtro, and tnfluoromethyl,
[0065](1) when R2 is H, p is 1 , R3 is methoxy, and R3 is at the b position of the nng, then R1 is other than methyl, ethyl, methoxy, ethoxy, tnfluoromethyl or phenyl,
[0066](m) when R1 is H, p is 1 , R3 is methoxy, and R3 is at the b position of the nng, then R2 is other than methyl, ethyl, methoxy, ethoxy, tnfluoromethyl or phenyl,
[0067](n) when R2 is H, p is 0 or 1 , R3 is chloro (when p is 1), and R3 is at the b position of the nng (when p is 1), then R1 is other than chloro, cyano, mtro, methyl, ethyl, isopropyl, methoxy, ethoxy, tnfluoromethyl, phenyl, methylthio, -C(O)O-(methyl), -C(O)-(methyl), -C(O)N-(methyl)2, -N(methyl)2, or benzyl,
[0068](o) when R1 is H, p is 0 or 1 , R3 is chloro (when p is 1), and R3 is at the b position of the nng (when p is 1), then R2 is other than chloro, cyano, mtro, methyl, ethyl, isopropyl, methoxy, ethoxy, tnfluoromethyl, phenyl, methylthio, -C(O)O-(methyl), -C(O)O-(ethyl), -C(O)-(methyl), -C(O)N-(methyl)2, -N(methyl)2, or benzyl,
[0069](p) when R1 is H, p is 2, each R3 is chloro, and the two R3 groups are at the b and d positions of the nng, then R2 is other than H,
20743-0032WO1 (AM103539; 44418, HTJBR- 1344 PCT) PATENT
[0070](q) when two R3 groups, together with the atoms to which they are attached, form an unsubstituted benzene πng, and R1 is H, then R2 is other than hydrogen, mtro, -C(O)O-(ethyl), -C(O)NHNH2, -C(O)NH2, -C(O)NH-(methyl), -C(O)NH-(phenyl), -C(0)NH-(cyclohexyl), -C(O)NH-(morpholm-l-yl), -C(O)NH-(pφeπdin-l-yl), -C(O)NH-(4-methylphenyl), -C(O)NH-(4-chlorophenyl), and -NH-C(O)O-(ethyl), and (r) when two R3 groups, together with the atoms to which they are attached, form an heterocyclo rmg, that heterocyclo πng is other than a 12-membered nng with 4 or more oxygen atoms
[0071]In some embodiments, p is 1, 2, or 3, R1 and R2 are other than H, and each R3 is independently selected from R4, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, -C(O)R4, - C(O)OR4, -O-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -Y1 -NR5R6, -Y1-N(R7)-Y2-NR8R9,
 and -P(O)(OR4)2
and -P(O)(OR4)2
[0072]In some embodiments, p is 1, 2, or 3 In some embodiments, p is 1 or 2 In some embodiments, p is 1 In some embodiments, p is 2
[0073]In some embodiments, R1 is selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, - C(O)R4, -C(O)OR4, -O-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -NR5R6, -C(O)NR5R6, -S(O)2-NR5R6, -N(R7)-C(O)-NR8R9, -N(R10)-C(O)-R4, and - N(R10)-C(O)O-R4, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl are each unsubstituted or substituted by one or more independently selected Z groups
[0074]In some embodiments, R1 is selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, - C(O)R4, -C(O)OR4, -O-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -NR5R6, -C(O)NR5R6, -S(O)2-NR5R6, -N(R7)-C(O)-NR8R9, -N(R10)-C(O)-R4, and - N(R10)-C(O)O-R4, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl are each unsubstituted or substituted by one or more independently selected Z groups, and wherein each R5, R6, R8, R9, and R10 is independently selected from H, alkyl, and haloalkyl
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0075]In some embodiments, R1 is selected from halo, alkyl, cycloalkyl, aryl, and heterocyclo, wherein said alkyl, cycloalkyl, aryl, and heterocyclo are each unsubstituted or substituted with one or more independently selected Z groups
[0076]In some embodiments, R1 is selected from alkyl, cycloalkyl, aryl, and heterocyclo, wherein said alkyl, cycloalkyl, aryl, and heterocyclo are each unsubstituted or substituted with one or more independently selected Z groups
[0077]In some embodiments, R1 is selected from alkyl, wherein said alkyl is unsubstituted or substituted with one or more independently selected Z groups
[0078]In some embodiments, R1 is selected from cycloalkyl, wherein said cycloalkyl is unsubstituted or substituted with one or more independently selected Z groups
[0079]In some embodiments, R1 is selected from aryl and heteroaryl, wherein said aryl and heteroaryl are each unsubstituted or substituted with one or more independently selected Z groups
[0080]In some embodiments, R1 is heterocyclo, which is unsubstituted or substituted with one or more independently selected Z groups
[0081]In some embodiments, R1 is heteroaryl, which is unsubstituted or substituted with one or more independently selected Z groups
[0082]In some embodiments, R1 is aryl, which is unsubstituted or substituted with one or more independently selected Z groups
[0083]In some embodiments, R1 is selected from bromo, ethyl, propyl, isobutyl, cyclohexyl, a phenyl ring, a thiophene πng, a pyrazole πng, an isooxazole πng, a thiazole ring, and a pyridine nng, wherein said ethyl, propyl, isobutyl, cyclohexyl, a phenyl πng, a thiophene πng, a pyrazole nng, an isooxazole πng, a thiazole πng, and a pyndme nng are each unsubstituted or substituted with one or more independently selected Z groups
[0084]In some embodiments, R1 is selected from H, bromo, ethyl, propyl, isobutyl, cyclohexyl, phenyl, thiophen-2-yl, lH-pyrazol-5-yl, lH-pyrazol-4-yl, furan-3-yl, isooxazol-4-yl, thiazol-5-yl, pyπdm-2-yl, pyndm-3-yl, and pyndm-4-yl, wherein said ethyl, propyl, isobutyl, cyclohexyl, phenyl, thiophen-2-yl, lH-pyrazol-5-yl, lH-pyrazol- 4-yl, isooxazol-4-yl, thiazol-5-yl, pyndm-2-yl, pyndm-3-yl, and pyndm-4-yl are each unsubstituted or substituted with one or more independently selected Z groups
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT
[0085]In some embodiments, R2 is selected from alkyl, cycloalkyl, cycloalkylalkyl, heterocyclo, and heterocycloalkyl, wherein alkyl, cycloalkyl, cycloalkylalkyl, heterocyclo, and heterocycloalkyl are each unsubstituted or substituted with one or more independently selected Z groups
[0086]In some embodiments, R2 is alkyl In some embodiments, R2 is methyl
[0087]In some embodiments, each R3 is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, -C(O)R4, -C(O)OR4, -0-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -NR5R6, -C(O)NR5R6, -S(O)2-NR5R6, -N(R7)-C(O)-NR8R9, -N(R10)- C(O)-R4, and -N(R10)-C(O)O-R4, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl are each unsubstituted or substituted by one or more independently selected Z groups
[0088]In some embodiments, each R3 is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, -C(O)R4, -C(O)OR4, -O-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -NR5R6, -C(O)NR5R6, -S(O)2-NR5R6, -N(R7)-C(O)-NR8R9, -N(R10)- C(O)-R4, and -N(R10)-C(O)O-R4, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl are each unsubstituted or substituted by one or more independently selected Z groups, and wherein each R5, R6, R8, R9, and R10 is independently selected from hydrogen, alkyl, and haloalkyl
[0089]In some embodiments, each R3 is independently selected from halo, cyano, mtro, - OH, -OR4, alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl are each unsubstituted or substituted with one or more independently selected Z groups
[0090]In some embodiments, each R3 is independently selected from halo, alkyl, -OH, - OR4, aryl, and heterocyclo, wherein said alkyl, aryl, and heterocyclo are each unsubstituted or substituted with one or more independently selected Z groups, and wherein each R4 is independently alkyl, haloalkyl, cycloalkylalkyl or aralkyl
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0091]In some embodiments, each R3 is independently selected from chloro, fluoro, - OH, tπfluoromethyl, methoxy, difluoromethoxy, cyclopropylmethoxy, pipendmyl, morpholmyl, piperazmyl, phenyl, lH-imidazol-1-yl, and benzyloxy, wherein said pipendinyl, moφhohnyl, piperazmyl, phenyl, and lH-imidazol-1-yl are are each unsubstituted or substituted with one or more independently selected Z groups
[0092]In some embodiments, each Z group is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR11, -SH, -SR11, -C(O)H, -C(O)OH, -C(O)R11, -C(O)OR11, -0-C(O)R11, -0-C(O)OR11, -SO3H, -S(O)11R1 ', halo, cyano, mtro, -NR12R13, -C(O)-NR12R13, -S(O)2-NR12R13, - OC(O)-NR12R13, -N(R14)-C(O)-NR15R16, -N(R17J-C(O)-R11, -N(R17)-C(O)O-RU, and oxo, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl is unsubstituted or substituted with one or more independently selected Z1 groups hi some embodiments, each Z group is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR11, -SH, -SR11, -C(O)H, -C(O)OH, -C(O)R11, -C(O)OR11, -0-C(O)R11, -0-C(O)OR11, -SO3H, -S(O)qRπ, halo, cyano, mtro, -NR12R13, -C(O)-NR12R13, -S(O)2-NR12R13, - OC(O)-NR12R13, -N(R14)-C(O)-NR15R16, -N(R17)-C(O)-RU, -N(R17J-C(O)O-R11, and oxo, wherein each R11 is independently alkyl or haloalkyl, and wherein each R12, R13, R14, R15, R16, and R17 is independently selected from hydrogen, alkyl, and haloalkyl
[0093]In some embodiments, each Z is independently selected from halo, cyano, mtro, alkyl, haloalkyl, cycloalkyl, -OH, -OR11, -SH, -SR11, -C(O)H, -C(O)OH, -C(O)R11, -C(O)OR11, -0-C(O)R11, -0-C(O)OR11, -SO3H, -S(O)qRH, halo, cyano, mtro, -NR12R13, - C(O)-NR12R13, -S(O)2-NR12R13, -OC(O)-NR12R13, -N(R14)-C(O)-NR15R16, -N(R17)- C(O)-R11, -N(R17J-C(O)O-R11, and oxo, wherein each R11 is independently alkyl or haloalkyl, and wherein each R12, R13, R14, R15, R16, and R17 is independently selected from H, alkyl, and haloalkyl
[0094]In some embodiments, each Z is independently selected from halo, cyano, mtro, alkyl, haloalkyl, alkoxy, haloalkoxy, cycloalkyl, -OH, -NR12R13, -C(O)-NR12R13, -S(O)2- NR12R13, -OC(O)-NR12R13, and -N(R17)-C(O)-Rπ, and oxo, wherein each R11 is
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT independently alkyl or haloalkyl, and wherein each R12, R13, and R17 is independently selected from H and alkyl
[0095]In some embodiments, each Z group is independently selected from halo, alkyl, haloalkyl, alkoxy, haloalkoxy, and -C(O)NR12R13
[0096]In some embodiments, each Z group is independently selected from chloro, fluoro, methyl, isopropyl, tπfluoromethyl, methoxy, ethoxy, isoproxy, n-propoxy, butoxy, tπfluoromethoxy, and -C(O)NHb
[0097]In some embodiments, each Z is independently selected from halo, alkyl, haloalkyl, alkoxy, and cycloalkyl
[0098]In some embodiments, each Z is independently selected from chloro, fluoro, methyl, isobutyl, tnfluoromethyl, ethoxy, propoxy, butoxy, and cyclohexyl
[0099]In some embodiments p is 1, 2, or 3,
[0100]R1 is selected from alkyl, aryl, aralkyl or heterocyclo, unsubstituted or substituted with one to three independently selected Z groups,
[0101]R2 is selected from alkyl, and each R3 is independently selected from -OH, -OR4, halo, cyano, nitro and -NR5R6, wherein Y1 represents a single bond
[0102]In some embodiments p is 1, 2, or 3,
[0103]R1 is selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, -C(O)R4, -C(O)OR4, -O- C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -NR5R6, -C(O)NR5R6, -S(O)2-NR5R6, -N(R7)-C(O)-NR8R9, -N(R10)-C(O)-R4, and -N(R10)-C(O)O-R4, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl are each unsubstituted or substituted by one or more independently selected Z groups,
[0104]R2 is selected from alkyl, cycloalkyl, cycloalkylalkyl, heterocyclo, and heterocycloalkyl, wherein alkyl, cycloalkyl, cycloalkylalkyl, heterocyclo, and heterocycloalkyl are each unsubstituted or substituted with one or more independently selected Z groups,
20743-0032WO1 (AM103S39; 44418; HUBR-1344 PCT) PATENT each R3 is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, - C(O)R4, -C(O)OR4, -0-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -NR5R6, -C(O)NR5R6, -S(O)2-NR5R6, -N(R7)-C(O)-NRSR9, -N(R10)-C(O)-R4, and - N(R10)-C(O)O-R4, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl are each unsubstituted or substituted by one or more independently selected Z groups, and each Z group is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR11, -SH, -SR11, -C(O)H, -C(O)OH, -C(O)R11, -C(O)OR11, -O-C(O)RU, -0-C(O)OR11, -SO3H, -S(O)qRπ, halo, cyano, mtro, -NR12R13, -C(O)-NR12R13, -S(O)2-NR12R13, -OC(O)-NR12R13, -N(R14)-C(O)-NR15R16, -N(R1^-C(O)-R1 ', -N(R17)-C(O)O-Rπ, and oxo, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl is unsubstituted or substituted with one or more independently selected Z1 groups
[0105]In some embodiments p is 1, 2, or 3,
[0106]R1 is selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, -C(O)R4, -C(O)OR4, -O-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -NR5R6, -C(O)NR5R6, -S(O)2-NR5R6, -N(R7)-C(O)-NR8R9, -N(R10)-C(O)-R4, and -N(R10)-C(O)O-R4, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl are each unsubstituted or substituted by one or more independently selected Z groups,
[0107]R2 is selected from alkyl, each R3 is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR4, -SH, -SR4, -C(O)H, -C(O)OH, - C(O)R4, -C(O)OR4, -O-C(O)R4, -0-C(O)OR4, -SO3H, -S(O)qR4, halo, cyano, mtro, -NR5R6, -C(O)NR5R6, -S(O)2-NR5R6, -N(R7)-C(O)-NR8R9, -N(R10)-C(O)-R4, and - N(R10)-C(O)O-R4, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl are each unsubstituted or substituted by one or more independently selected Z groups,
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT each Z group is independently selected from alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, heterocycloalkyl, -OH, -OR11, -SH, -SR11, -C(O)H, -C(O)OH, -C(O)R11, -C(O)OR11, -O-C(O)RU, -O-C(O)ORΠ, -SO3H, -S(O)qRu, halo, cyano, mtro, -NR12R13, -C(O)-NR12R13, -S(O)2-NR12R13, -OC(O)-NR12R13, -N(R14)-C(O)-NR15R16, -N(R17)-C(O)-RU, -N(R17)-C(O)O-Rn, and oxo, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl is unsubstituted or substituted with one or more independently selected Z1 groups, each R11 is independently alkyl or haloalkyl, and each R5, R6, R8, R9, R10, R12, R13, R14, R15, R16, and R17 is independently selected from H, alkyl, and haloalkyl
[0108]In some embodiments p is 1, 2, or 3,
[0109]R1 is selected from halo, alkyl, cycloalkyl, aryl, and heteroaryl, wherein said alkyl, cycloalkyl, aryl, and heteroaryl are each unsubstituted or substituted with one or more independently selected Z groups,
[0110]R2 is selected from alkyl, each R3 is independently selected from halo, cyano, mtro, -OH, -OR4, alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl are each unsubstituted or substituted with one or more independently selected Z groups, each Z is independently selected from halo, cyano, mtro, alkyl, haloalkyl, alkoxy, haloalkoxy, cycloalkyl, -OH, -OR11, -SH, -SR11, -C(O)H, -C(O)OH, -C(O)R11, -C(O)OR11, -O-C(O)RU, -0-C(O)OR11, -SO3H, -S(O)qRn, halo, cyano, mtro, -NR12R13, -C(O)-NR12R13, -S(O)2-NR12R13, -OC(O)-NR12R13, -N(R14)-C(O)-NR15R16, -N(R17)- C(O)-R11, -N(R17)-C(O)O-RU, and oxo, each R11 is independently alkyl or haloalkyl, and each R12, R13, R14, R15, R16, and R17 is independently selected from H, alkyl, and haloalkyl
[0111]In some embodiments p is 1, 2, or 3,
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0112]R1 is selected from alkyl, cycloalkyl, aryl, and heteroaryl, wherein said alkyl, cycloalkyl, aryl, and heteroaryl are each unsubstituted or substituted with one or more independently selected Z groups,
[0113]R2 is selected from alkyl, each R3 is independently selected from halo, cyano, mtro, -OH, -OR4, alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl are each unsubstituted or substituted with one or more independently selected Z groups, each Z is independently selected from halo, cyano, mtro, alkyl, haloalkyl, alkoxy, haloalkoxy, cycloalkyl, -OH, -NR12R13, -C(O)-NR12R13, -S(O)2-NR12R13, -OC(O)-NR12R13, and -N(R1^-C(O)-R1 \ and oxo, each R11 is independently alkyl or haloalkyl, and each R12, R13, and R17 is independently selected from H, alkyl, and haloalkyl
[0114]In some embodiments p is 1, 2, or 3,
[0115]R1 IS alkyl or cycloalkyl, which are each unsubstituted or substituted with one or more independently selected Z groups,
[0116]R2 is selected from alkyl, each R3 is independently selected from halo, cyano, mtro, -OH, -OR4, alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl, wherein said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl are each unsubstituted or substituted with one or more independently selected Z groups, each Z is independently selected from halo, cyano, mtro, alkyl, haloalkyl, alkoxy, haloalkoxy, cycloalkyl, -OH, -NR12R13, -C(O)-NR12R13, -S(O)2-NR12R13, -OC(O)-NR12R13, and -N(R17)-C(O)-RU, and oxo, each R11 is independently alkyl or haloalkyl, and each R12, R13, and R17 is independently selected from H, alkyl, and haloalkyl
[0117]In some embodiments p is 1 , 2, or 3 ,
[0118]R1 is aryl or heteroaryl, which are each unsubstituted or substituted with one or more independently selected Z groups,
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0119]R2 is selected from alkyl, each R3 is independently selected from halo, cyano, mtro, -OH, -OR4, alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl, wherem said alkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heterocyclo, and heterocycloalkyl are each unsubstituted or substituted with one or more independently selected Z groups, each Z is independently selected from halo, cyano, mtro, alkyl, haloalkyl, alkoxy, haloalkoxy, cycloalkyl, -OH, -OR11, -NR12R13, -C(O)-NR12R13, -S(O)2-NR12R13, -OC(O)-NR12R13, and -N(R17J-C(O)-R1 \ and oxo, each R11 is independently alkyl or haloalkyl, and each R12, R13, and R17 is independently selected from H, alkyl, and haloalkyl
[0120]In some embodiments p is 1, 2, or 3,
[0121]R1 is selected from H, bromo, ethyl, propyl, isobutyl, cyclohexyl, a phenyl πng, a thiophene πng, a pyrazole πng, an isooxazole πng, a thiazole nng, and a pyridine πng, wherein said ethyl, propyl, isobutyl, cyclohexyl, a phenyl nng, a thiophene πng, a pyrazole nng, an isooxazole nng, a thiazole nng, and a pyndine nng are each unsubstituted or substituted with one or more Z groups independently selected from halo, alkyl, haloalkyl, alkoxy, haloalkoxy, and -C(O)NR12R13
[0122]R2 is selected from alkyl, each R3 is independently selected from halo, alkyl, -OH, -OR4, aryl, and heterocyclo, wherein said alkyl, aryl, and heterocyclo are each unsubstituted or substituted with one or more Z groups independently selected from halo, alkyl, haloalkyl, alkoxy, and cycloalkyl, each R12 and R13 is independently selected from H and alkyl
[0123]In some embodiments p is 1, 2, or 3,
[0124]R1 is selected from H, bromo, ethyl, propyl, isobutyl, cyclohexyl, phenyl, thiophen-2-yl, lH-pyrazol-5-yl, lH-pyrazol-4-yl, isooxazol-4-yl, thiazol-5-yl, pyndin-2- yl, pyπdm-3-yl, and pyπdm-4-yl, wherein said ethyl, propyl, isobutyl, cyclohexyl, phenyl, thiophen-2-yl, lH-pyrazol-5-yl, lH-pyrazol-4-yl, isooxazol-4-yl, thiazol-5-yl, pyπdm-2-yl, pyndm-3-yl, and pyndin-4-yl are each unsubstituted or substituted with one
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT or more groups independently selected from chloro, fluoro, methyl, isopropyl, tnfluoromethyl, methoxy, ethoxy, isoproxy, n-propoxy, butoxy, tπfluoromethoxy, and -C(O)NH2,
[0125]R2 is methyl, and each R3 is independently selected from chloro, fluoro, -OH, tnfluoromethyl, methoxy, difluoromethoxy, cyclopropylmethoxy, prpendmyl, morpholmyl, piperazinyl, phenyl, lH-imidazol-1-yl, and benzyloxy, wherein said pipeπdmyl, morpholmyl, piperazinyl, phenyl, and lH-imidazol-1-yl are are each unsubstituted or substituted with one or more groups independently selected from chloro, fluoro, methyl, isobutyl, tnfluoromethyl, ethoxy, propoxy, butoxy, and cyclohexyl
[0126]In some embodiments, the compound is a compound of Formula Ib
[0127] or a pharmaceutically acceptable salt thereof
or a pharmaceutically acceptable salt thereof
[0128]In some embodiments, the compound is a compound of Formula Ic
[0129] or a pharmaceutically acceptable salt thereof
or a pharmaceutically acceptable salt thereof
[0130]The present invention further provides a compound of formula (Ia)
[0131]
[0132]Ia
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT wherein p is 0 or an integer from 1 to 4, each R1, and R2, and R3, are independently selected from hydrogen or R4, where R4 is alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, cycloalkenylalkyl, aryl, aralkyl, heterocyclo, or heterocycloalkyl, each of which is unsubstituted or substituted with one or more (preferably, one to three) groups Z,
[0134]-SH or -SR4, -C(O)qH, -C(O)qR4, or -O-C(O)qR4, where q is 1 or 2,
[0135]-SO3H or -S(O)qR4 halo, cyano, nitro, -Y'-NR5R6,
[0136]-Y'-N(R7)-Y2-NR8R9,
[0138]-P(O)(OR4)2, any two groups R3 may together be alkylene or alkenylene completing a 3- to 8- membered saturated or unsaturated nng together with the carbon atoms to which they are attached, which nng is unsubstituted or substituted with one or more groups Z, or any two groups of R3 may, together with the atoms to which they are attached, form a heterocyclo group which is unsubstituted or substituted with one or more groups Z, R5, R6, R7, Rs, R9 and R10, are independently hydrogen or R4,
[0139]R5 and R6 may together be alkylene or alkenylene, completing a 3- to 8- membered saturated or unsaturated nng with the nitrogen atom to which they are attached, which nng is unsubstituted or substituted with one or more groups Z, any two of R7, R8 and R9 may together be alkylene or alkenylene, completing a 3- to 8-membered saturated or unsaturated nng with the nitrogen atom to which they are attached, which nng is unsubstituted or substituted with one or more groups Z,
20743-0032WO1 (AM103539, 44418, HUBR- 1344 PCT) PATENT
[0140]Z groups are each independently hydrogen or R11, where R11 is alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkylalkyl, cycloalkenyl, cycloalkenylalkyl, aryl, aralkyl, heterocyclo, or heterocycloalkyl, each of which is unsubstituted or substituted with one or more (preferably, one to three) groups Z1,
[0141]-OH or -OR11, -SH or -SR11,
[0142]-C(O)qH, -C(O)qRn, Or-O-C(O)C1R11, where q is 1 or 2, -SO3H or -S(O)11R1 ! halo, cyano, mtro,
[0143]-Y^NR5R6, -Y'-N(R7)-Y2-NR8R9, -Y'-NCR^-Y^R11,
[0146]Y1 and Y2 are each independently a single bond, -Y3-S(O)q-Y4-,
[0150]-Y3-S-Y4-, -Y3-O-C(O)-Y4-, or
[0152]Y3 and Y4 are each independently a single bond, alkylene, alkenylene, or alkynylene
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0153]In some embodiments, p is selected from 1, 2 or 3
[0154]In some embodiments, R1 is selected from alkyl, aryl, aralkyl or heterocyclo, unsubstituted or substituted with one to three groups Z
[0155]In some embodiments, R2 is selected from hydrogen or alkyl In some embodiments, each R3 is independently selected from hydrogen, -OH, -
[0156]OR4, halo, cyano, mtro or -Yl-NR5R6 (wherein Y1 represents a single bond) In some embodiments, the compound is selected from 8-fluoro-3-methyl-l-propyl-imidazo[5,l-c]-l,2,4-benzotnazine, l-ethyl-8-fluoro-3-methyl-imidazo[5,l-c]-l,2,4-benzotriazine, l-cyclohexyl-S-fluoro-S-methyl-imidazofSjl-cj-l^^-benzotπazme, l-(2,5-dichlorophenyl)-8-methoxy-3-methyl-imidazo[5,l-c]-l,2,4-benzotnazme, l-(2,5-dichlorophenyl)-7-fluoro-3-methyl-imidazo[5,l-c]-l,2,4-benzotπazine, and
[0157]1 -(2,5-dichlorophenyl)-7-methoxy-3-methyl-imidazo[5, 1 -c]-l ,2,4-benzotπazme, or a pharmaceutically acceptable salt thereof
[0158]In some embodiments, the compound is selected from
[0159]8-fluoro-3 -methyl- 1 -propyl-imidazo [ 5 , 1 -c] [ 1 ,2,4]benzotnazine,
[0160]7-methoxy-3-methyl-l-propyl-imidazo[5,l-c][l,2,4]benzotnazine,
[0161]1 -ethyl-8-fluoro-3-methyl-imidazo [5, 1 -c] [ 1 ,2,4]benzotπazme, l-cyclohexyl-8-fluoro-3-methyl-imidazo[5,l-c][l,2,4]benzotπazme,
[0162]1 -(2, 5 -dichlorophenyl)-8 -methoxy-3 -methyl-imidazo [ 5 , 1 -c] [ 1 ,2,4]benzotnazine, l-(2,5-dichlorophenyl)-7-fluoro-3-methyl-imidazo[5,l-c][l,2,4]benzotnazine, and l-(2,5-dichlorophenyl)-7-methoxy-3-methyl-imidazo[5,l-c][l,2,4]benzotπazine, or a pharmaceutically acceptable salt thereof
[0163]In some embodiments, the compound is selected from l-(2,5-dichlorophenyl)-8-fluoro-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazine,
[0164]8-fluoro-l-isobutyl-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazme, l-sec-butyl-8-fluoro-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tnazine,
[0165]8-fluoro-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tnazme,
[0166]8-fluoro-3-methyl-l-(2-methylpyridin-3-yl)benzo[e]imidazo[5,l-c][l,2,4]tnazme, l-(2-chlorophenyl)-8-fluoro-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tnazme,
[0167]1 -(2,3-dichlorophenyl)-8-fluoro-3-methylbenzo[e]imidazo[5, 1 -c] [1 ,2,4]tπazme,
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0168]8-fluoro-3-methyl-l-(l-methyl-lH-pyrazol-5-yl)benzo[e]imidazo[5,l- c][l,2,4]tnazine,
[0169]8-fluoro-l-(2-methoxyphenyl)-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazine, 8-fluoro-3 -methyl- 1 -o-tolylbenzo[e]imidazo[5, 1 -c] [1 ,2,4]tnazine, l-(2-chloro-5-methoxyphenyl)-8-fluoro-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tnazme, l-(4-chloropyndm-3-yl)-8-fluoro-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazme, 8-fluoro-l-(2-fluoro-5-isopropoxyphenyl)-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tnazine,
[0170]8-fluoro-l-(2-fluoro-5-(tπfluoromethyl)phenyl)-3-methylbeπzo[e]imidazo[5,l- c][l,2,4]tnazine, l-(5-butoxy-2-fluorophenyl)-8-fluoro-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tnazine,
[0171]8-fluoro-l-(2-fluoro-5-propoxyphenyl)-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tnazine, l-(5-ethoxy-2-fluorophenyl)-8-fluoro-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tnazine,
[0172]7-fluoro-3-methyl-l-(2-methylpyπdm-3-yl)benzo[e]imidazo[5,l-o][l,2,4]tπazme,
[0173]7-fluoro-l-(2-methoxyphenyl)-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazme,
[0174]7-fluoro-l-(4-fluoro-2-methylphenyl)-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tπazme,
[0175]7-fluoro-3 -methyl- 1 -o-tolylbenzo [e]imidazo[5, 1 -c] [ 1 ,2,4]tnazine, l-(2-chloro-5-methoxyphenyl)-7-fluoro-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tnazine,
[0176]7-fluoro-l-(2-fluoro-5-(tnfluoromethyl)phenyl)-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tnazme,
[0177]7-fluoro-l-(2-fluoro-5-isopropoxyphenyl)-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tπazme, l-(5-butoxy-2-fluorophenyl)-7-fluoro-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tπazme,
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0178]7-fluoro-l-(2-fIuoro-5-propoxyphenyl)-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tπazme,
[0179]1 -(2-chlorophenyl)-7-methoxy-3-methylbenzo[e]imidazo[5,l -c] [ 1 ,2,4]tπazine,
[0180]7-methoxy-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tnazine,
[0181]7-methoxy-3-methyl-l-(2-methylpyπdin-3-yl)benzo[e]unidazo[5,l- c][l,2,4]tπazine,
[0182]8-chloro-l-(2,5-dichlorophenyl)-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tnazine, 8-chloro-3-methyl-l-(2-methylpyndm-3-yl)benzo[e]imidazo[5,l-c][l,2,4]tnazme, 8-chloro-l-(2-methoxyphenyl)-3-methylbenzo[e]πmdazo[5,l-c][l,2,4]tnazme, 8-chloro-3-methyl-l-(l-methyl-lH-pyrazol-5-yl)benzo[e]imidazo[5,l- c][l,2,4]tπazine,
[0183]2-(8-chloro-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazin-l-yl)benzamide, 8-chloro-3-methylbenzo[e]imidazo[5, 1 -c] [1 ,2,4]tnazine, 8-chloro-l-(2-fluoro-5-isopropoxyphenyl)-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tnazme, l-(2-fluoro-5-isopropoxyphenyl)-8-methoxy-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tnazine,
[0184]6,8-dimethoxy-3-methyl-l-(4-methylpyπdm-3-yl)imidazo[5>l- c] [1 ,2,4]benzotπazme,
[0185]1 -(2-chlorophenyl)-7, 8 -dimethoxy-3 -methylb enzo [e] lmidazo [5 , 1 - c][l,2,4]tnazine,
[0186]6,8-dimethoxy-3 -methyl-1 -(3 -methylpyπdm-4-yl)imidazo[5, 1 - c] [ 1 ,2,4]benzotπazme,
[0187]6,8-dimethoxy-3-methyl-l-(2-methylpyndin-3-yl)imidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0188]7,8-dimethoxy-3-methyl-l-o-tolylbenzo[e]imidazo[5,l-c][l,2,4]tnazme,
[0189]7,8-dimethoxy-3-methyl-l-(pyπdin-2-yl)benzo[e]imidazo[5,l-c][l,2,4]tnazine, l-CS.S-dimethyl-lH-pyrazol^-yO-ό.S-dimethoxy-S-methylimidazofS,!- c] [ 1 ,2,4]benzotπazme,
[0190]6,8-dimethoxy-3-methyl- 1 -(1 ,3,5-tnmethyl-lH-pyrazol-4-yl)imidazo[5, 1 - c] [ 1 ,2,4]benzotπazme,
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT l-isobutyl-7,8-dimethoxy-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazme, l-bromo^S-dimethoxy-S-methylbenzofelimidazo^^-cJllA^tnazme, l-(2,5-dichlorophenyl)-7,8-dimethoxy-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tπazme, l-(2-chloro-5-methylρhenyl)-7,8-dimethoxy-3-methylbenzo[e]πnidazo[5,l- c][l,2,4]tπazme,
[0191]7,8-dimethoxy-3-methyl-l-(2-(tnfluoromethyl)ρhenyl)benzo[e]iπudazo[5,l- c][l,2,4]tπazme, l-(2-chloroρhenyl)-7,8-difluoro-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazme,
[0192]7,8-difluoro-l-isobutyl-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazine,
[0193]6-methoxy-3-methyl-l-(2-methylphenyl)imidazo[5,l-c][l,2,4]benzotπazme,
[0194]1 -(2-chlorophenyl)-6-methoxy-3-methylbenzo[e]imidazo[5, 1 -c][ 1 ,2,4]tπazme,
[0195]6-methoxy-3-methyl-l-(3-methylpyπdm-4-yl)imidazo[5,l-c][l,2,4]benzotπazme,
[0196]6-methoxy-3-methyl-l-(3-methylpyπdm-4-yl)-8-morpholm-4-ylimidazo[5,l- c] [ 1 ,2,4]benzotxiazme,
[0197]6-methoxy-3-methyl-l-(2-methylpyπdm-3-yl)-8-morpholm-4-ylimidazo[5,l- c] [ 1 ,2,4]benzotπazine l-(2-chlorophenyl)-6-methoxy-3-methyl-8-morpholin-4-ylimidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0198]6-methoxy-3-methyl-l-(2-methylphenyl)-8-morpholin-4-ylimidazo[5,l- c] [ 1 ,2,4]benzotπazine,
[0199]6-fluoro-8-methoxy-l-(3-methoxyphenyl)-3-methyliinidazo[5,l- c] [ 1 ,2,4]benzotnazine, l-(5-chloro-2-methoxyphenyl)-6-fluoro-8-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotπazine,
[0200]6-fluoro-l-(4-fluoro-2-methylphenyl)-8-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotπazine, l-(2-chloro-4-fluorophenyl)-6-fluoro-8-methoxy-3-methyhmidazo[5,l- c] [ 1 ,2,4]benzotπazine,
[0201]1 -(2-chloro-4-methylphenyl)-6 -fluoro- 8 -methoxy- 3 -methyhmidazo [5,1- c] [ 1 ,2,4]benzotπazine,
20743-0032WO1 (AM103539; 44418; H€BR-1344 PCT) PATENT
[0202]1 -(l-chloro-S-methylphenylJ-δ-fluoro-S-methoxy-S-methylimidazoCS, 1 - c] [ 1 ,2,4]benzotnazme,
[0203]6-fluoro-l-(5-fluoro-2-methylphenyl)-8-methoxy-3-mefliylimidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0204]6-fluoro-l-(2-fluoro-4-methylρhenyl)-8-methoxy-3-methylinudazo[5,l- c][l,2,4]benzotπazme,
[0205]6-fluoro-l-(2-fluoro-5-methylphenyl)-8-methoxy-3-methylmudazo[5,l- c] [1 ,2,4]benzotπazme,
[0206]6-fluoro-l-(2-fluoro-5-methoxyphenyl)-8-methoxy-3-methylimidazo[5,l- c][l,2,4]benzotπazme, l-(2-chloro-5-ethoxyphenyl)-6-fluoro-8-methoxy-3-methyhmidazo[5,l- c][l ,2,4]benzotnazme,
[0207]1 -(2-chloro-5-methoxyphenyl)-6-fluoro-8-methoxy-3 -methyhmidazo[5, 1 - c] [ 1 ,2,4]benzotnazme,
[0208]1 -(5-chloro-2-methylphenyl)-6-fluoro-8-methoxy-3-methylimidazo[5, 1 - c] [ 1 ,2,4]benzotπazme, l-(2-chloro-5-fluorophenyl)-6-fluoro-8-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0209]6-fluoro-8-methoxy-3-methyl-l-(3-methylthiophen-2-yl)imidazo[5,l- c] [ 1 ,2,4]benzotπazme, l-[2-chloro-5-(txifluoromethyl)phenyl]-6-fluoro-8-methoxy-3-methyliinidazo[5,l- c] [ 1 ,2,4]benzotnazine, l-[2-chloro-5-(fxifluoromethoxy)phenyl]-6-fluoro-8-methoxy-3- methylimidazo[5, 1 -c] [ 1 ,2,4]benzotπazine,
[0210]6-fluoro-l-(3-fluoro-2-methylphenyl)-8-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotπazme, δ-fluoro-S-methoxy-S-methyl-l-CljS^-tπmethyl-lH-pyrazol^-ylJimidazoCS,!- c] [ 1 ,2,4]benzotπazme,
[0211]6-fluoro-8-methoxy-l-(2-methoxyρhenyl)-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotπazine,
[0212]6-fluoro-8-methoxy-3-methyl-l-pyndm-4-ylimidazo[5,l-c][l,2,4]benzotπazme,
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT l-(5-chloro-2-fluorophenyl)-6-fluoro-8-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotnazme, l-(3,5-dimethylisoxazol-4-yl)-6-fluoro-8-methoxy-3-methyhmidazo[5,l- c] [ 1 ,2,4]benzotnazme,
[0213]6-fluoro-8-methoxy-3-methyl-l-pyπdm-3-ylimidazo[5,l-c][l,2,4]benzotπazme,
[0214]1 -(2,4-dimethyl- 1 ,3 -thiazol-5-yl)-6-fluoro-8-methoxy-3 -methyhmidazo[5 , 1 - c] [ 1 ,2,4]benzotnazme,
[0215]6-fluoro-l-(6-fluoro-5-methylpyndm-3-yl)-8-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotnazine,
[0216]6-fluoro- 1 -(6-fluoro-2-methylpyndm-3-yl)-8-methoxy-3 -methyhmidazo[5, 1 - c] [ 1 ,2,4]benzotπazme,
[0217]6-fluoro-l-(2-fluoroρyπdm-3-yl)-8-methoxy-3-methylimidazo[5,l- c][l,2,4]benzotπazme,k
[0218]6-fluoro-8-methoxy-l-(5-methoxypyndin-3-yl)-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotnazme, l-(3,5-dmiethyl-lH-pyrazol-4-yl)-6-fluoro-8-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0219]6-fluoro-8-methoxy-3-methyl-l-(4-methylpyndin-3-yl)imidazo[5,l- c] [ 1 ,2,4]benzotnazme,
[0220]4-fluoro-3-(6-fluoro-8-methoxy-3-methylimidazo[5,l-c][l,2,4]benzotnazin-l- yljbenzamide,
[0221]8-fluoro-6-methoxy- 1 -(3-methoxyphenyl)-3-methyhmidazo[5, 1 - c] [ 1 ,2,4]benzotnazme,
[0222]8-fluoro-6-methoxy- 1 -(2-methoxyphenyl)-3-methyhmidazo [5,1- c] [ 1 ,2,4]benzotnazme, l-[2-chloro-5-(tπfluoromethyl)phenyl]-8-fluoro-6-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotnazme, l-(5-chloro-2-methoxyphenyl)-8-fluoro-6-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotπazine,
[0223]8 -fluoro- 1 -(5 -fluoro -2-methylphenyl) -6-methoxy-3 -methyhmidazo [5,1- c] [ 1 ,2,4]benzotnazine,
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT l-(5-chloro-2-methylphenyl)-8-fluoro-6-methoxy-3-methyhmidazo[5,l- c] [ 1 ,2,4]benzotπazine,
[0224]8-fluoro-l-(2-fluoro-5-methylphenyl)-6-methoxy-3-methyhmidazo[5,l- c] [ 1 ,2,4]benzotπazme, l-(5-chloro-2-fluorophenyl)-8-fluoro-6-methoxy-3-methyliinidazo[5,l- c] [ 1 ,2,4]benzotπazme, l-(2-cMoro-5-methylphenyl)-8-fluoro-6-methoxy-3-methyhmidazo[5,l- c] [ 1 ,2,4]benzotnazme, l-(2-chloro-5-ethoxyphenyl)-8-fluoro-6-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotnazine,
[0225]S-fluoro-δ-methoxy-S-methyl-l-pyπdm-S-ylimidazofS.l-clfl^^Jbenzotπazine,
[0226]8-fluoro-l-(4-fluoro-2-methylphenyl)-6-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotπazme, l-(2-chloro-4-fluorophenyl)-8-fluoro-6-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotπazme, l-(2-chloro-4-methylphenyl)-8-fluoro-6-methoxy-3-methyhmidazo[5,l- c][l,2,4]benzotnazme,
[0227]8-fluoro-l-(2-fluoro-5-methoxyphenyl)-6-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotπazine,
[0228]8-fluoro-l-(2-fluoro-4-methylphenyl)-6-methoxy-3-methyliimdazo[5,l- c] [ 1 ,2,4]benzotπazme, l-(3,5-dimethylisoxazol-4-yl)-8-fluoro-6-methoxy-3-methyhmidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0229]8-fluoro-6-methoxy-3-methyl-l-(l,3,5-tπmethyl-lH-pyrazol-4-yl)imidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0230]8-fluoro-l-(3-fluoro-2-methylphenyl)-6-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotnazme, l-(2-chloro-5-fluorophenyl)-8-fluoro-6-methoxy-3-methyhmidazo[5,l- c] [ 1 ,2,4]benzotnazme,
[0231]8-fluoro-6-methoxy-3 -methyl- 1 -(3-methylthiophen-2-yl)imidazo[5, 1 - c] [ 1 ,2,4]benzotπazme,
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0232]8-fluoro-l-(6-fluoro-2-methylpyndin-3-yl)-6-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotnazme,
[0233]8-fluoro-l-(6-fluoro-5-methylpyndin-3-yl)-6-methoxy-3-methylimidazo[5,l- c][l,2,4]benzotnazine,
[0234]8-fluoro-6-methoxy-l -(5-methoxypyπdin-3-yl)-3-methylimidazo[5, 1 - c] [ 1 ,2,4]benzotnazine,
[0235]8-fluoro-6-methoxy-3-methyl-l-(4-πiethylpyπdm-3-yl)imidazo[5,l- c] [ 1 ,2,4]benzotnazine, l-(3,5-dimethyl-lH-pyrazol-4-yl)-8-fluoro-6-methoxy-3-methylimidazo[5,l- c] [ 1 ,2,4]benzotnazine, l-[2-chloro-5-(tπfluoromethoxy)phenyl]-8-fluoro-6-methoxy-3- methylimidazo[5,l-c][l,2,4]benzotπazine, l-(2-chloro-5-methoxyphenyl)-8-fluoro-6-methoxy-3-methyhimdazo[5,l- c] [ 1 ,2,4]benzotnazme, l-(2,4-dimethyl-l,3-thiazol-5-yl)-8-fluoro-6-methoxy-3-mefhylimidazo[5,l- c][l,2,4]benzotnazme,
[0236]4-fluoro-3-(8-fluoro-6-methoxy-3 -methylimidazo[5, 1 -c] [ 1 ,2,4]benzotnazm- 1 - yl)benzamide,
[0237]8-fluoro-6-methoxy-3-methyl- 1 -pyπdm-4-ybmidazo[5, 1 -c] [ 1 ,2,4]benzotπazme,
[0238]8-fluoro-6-methoxy-3-methyl-l-(2-methylpyπdin-3-yl)imidazo[5,l- c] [ 1 ,2,4]benzotπazine,
[0239]8-fluoro-6-methoxy-3-methyl-l -(3-methylpyndm-4-yl)imidazo[5, 1 - c] [ 1 ,2,4]benzotπazme,
[0240]6-Chloro-3-methyl-l-(3-methylpyπdin-4-yl)-8-(tπfluoromethyl)imidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0241]6-chloro-l-(2,5-dichlorophenyl)-3-methyl-8-(tπfluoromethyl)imidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0242]6-methoxy-3-methyl-l-(3-methylpyndm-4-yl)-8- (tπfluoromethyl)benzo [e] imidazo [5 , 1 -c] [ 1 ,2,4] tnazine, l-(2-Chlorophenyl)-6-methoxy-3-methyl-8-(tnfluoromethyl)imidazo[5,l- c] [ 1 ,2,4]benzotπazine,
20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT
[0243]6-Methoxy-3-methyl-l-(2-methylpyndm-3-yl)-8-(tπfluoromethyl)imidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0244]6-Methoxy-3-methyl-l-(4-methylpyndm-3-yl)-8-(tπfIuoromethyl)imidazo[5,l- c] [ 1 ,2,4]benzotπazme, ό-Methoxy-S-methyl-l-CS-methylthiophen^-yO-S-Ctnfluoromethy^imidazofS,!- c] [ 1 ,2,4]benzotnazme,
[0245]6-Mefhoxy-l-(3-methoxypyndin-4-yl)-3-methyl-8-(tnfluoromethyl)benzo[e] imidazo[5, 1 -c] [ 1 ,2,4]tπazme, l-(2,5-Dichlorophenyl)-6-methoxy-3-methyl-8- (tπfluoromethyl)benzo[e]imidazo[5,l-c][l,2,4]tπazme, l-(3-Fluoro-2-methylphenyl)-6-methoxy-3-methyl-8-(tπfluoromethyl)benzo[e] imidazo[5,l-c][l,2,4]tπazme,
[0246]^(S-Chloro^-methoxypheny^-δ-methoxy-S-methyl-S-^fluoromethy^benzoCe] imidazo[5, 1 -c] [ 1 ,2,4] tπazine, l-(Furan-3-yl)-6-methoxy-3-methyl-8-(tπfluoromethyl)benzo[e]iπudazo[5,l- c][l,2,4]tπazme,
[0247]4-(6-Methoxy-3-methyl-8-(tπfluoromethyl)benzo[e]imidazo[5,l-c][l,2,4]tnazm- 1 -yl)-3,5-dimethybsoxazole, ό-Methoxy-S-methyl-l-Cthiophen^-yy-S-Ctπfluoromethy^benzofelimidazo [5,1- c][l,2,4]tnazme, l-(5-Fluoro-2-methylphenyl)-6-methoxy-3-methyl-8-(tnfluoromethyl)benzo[e] imidazo[5,l-c][l,2,4]tnazme,
[0248]6-methoxy-3-methyl-l-(4-methylpyndin-3-yl)benzo[e]imidazo[5,l- c][l,2,4]tπazm-8-ol,
[0249]8-(difluoromethoxy)-6-methoxy-3-methyl-l-(4-methylpyndm-3- yl)benzo[e]imidazo[5,l-c][l,2,4]tnazine,
[0250]8-(benzyloxy)-6-methoxy-3-methyl-l-(3-methylpyπdm-4-yl)imidazo[5,l- c] [ 1 ,2 ,4]b enzotnazme,
[0251]6-methoxy-3-methyl-l-(3-methylpyπdm-4-yl)benzo[e]imidazo[5,l- c][l,2,4]tπazm-8-ol,
20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT
[0252]8-(difluoromethoxy)-6-methoxy-3-methyl-l-(3-methylpyπdm-4-yl)imidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0253]8-(benzyloxy)-6-methoxy-3-methyl-l-(2-methylpyndin-3-yl)imidazo[5,l- c] [ 1 ,2,4]benzotnazine,
[0254]6-methoxy-3-methyl- 1 -(2-methylpyπdin-3 -yl)imidazo[5, 1 -c] [ 1 ,2,4]benzotnazin- 8- ol,
[0255]8-(difluoromethoxy)-6-methoxy-3-methyl-l-(2-methylρyndin-3-yl)imidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0256]8-(benzyloxy)-6-methoxy-3-methyl-l-(4-methylρyndm-3-yl)miidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0257]8-(cyclopropylmethoxy)-6-methoxy-3-methyl-l-(3-methylpyndm-4- yl)benzo[e]imidazo[5, 1 -c] [132,4]tπazme,
[0258]8-(cyclopropylmethoxy)-6-methoxy-3-methyl-l-(2-methylpyπdm-3- yl)benzo[e]imidazo[5, 1 -c] [1 ,2,4]tnazme,
[0259]8-(difluoromethoxy)-6-methoxy-3-methyl-l-(l,3,5-tπmethyl-lH-pyrazol-4- yl)benzo[e]imidazo[5, 1 -c][l ,2,4]tnazine,
[0260]S-(difluoromethoxy)- 1 -(3 ,5-dimethyl- 1 H-pyrazol-4-yl)-6-methoxy-3 - methylbenzo[e]imidazo[5, 1 -c][ 1 ,2,4]tnazine,
[0261]4-(8-(difluoromethoxy)-6-methoxy-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tnazm- 1 -yl)-3,5-dimethyhsoxazole,
[0262]5-(8-(difluoromethoxy)-6-methoxy-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tnazm- 1 -yl)-2,4-dimethylthiazole,
[0263]8-(difluoromethoxy)-l-(3-fluoro-2-methylphenyl)-6-methoxy-3- methylbenzo [e] imidazo [5 , 1 -c] [ 1 ,2,4]tπazme,
[0264]8-(difluoromethoxy)-l-(5-fluoro-2-methylphenyl)-6-methoxy-3- methylbenzo[e]imidazo[5, 1-c] [ 1 ,2,4]tπazme, l-(2-chloro-5-fluorophenyl)-8-(difluoromethoxy)-6-methoxy-3- methylbenzo[e]imidazo[5, 1 -c] [ 1 ,2,4]tπazme, l-(5-chloro-2-methoxyphenyl)-8-(difluoromethoxy)-6-methoxy-3- methylbenzo[e]imidazo[5, 1 -c] [1 ,2,4]tnazme,
20743-0032WO1 (AM103539, 44418, HTJBR-1344 PCT) PATENT
[0265]1 -(2,5-dichlorophenyl)-8-(difluoromethoxy)-6-methoxy-3-methylimidazo[5, 1 - c] [ 1 ,2,4]benzotπazme, l-(2-chloroρhenyl)-8-(difluoromethoxy)-6-methoxy-3-methyhmidazo[5,l- c] [ 1 ,2,4]benzotnazme,
[0266]8-(difluoromethoxy)-6-methoxy-l-(5-methoxypyndm-3-yl)-3-methyhmidazo[5,l- c] [ 1 ,2,4]benzotπazme, l,8-Bis-(2,5-dichloro-phenyl)-3-methyl-imidazo[5,l-c][l,2,4]benzotπazme, l,8-Bis-(2-chloro-phenyl)-3-methyl-imidazo[5,l-c][l,2,4]benzotπazme, l,8-Bis-(2,3-dichloro-phenyl)-3-methyl-imidazo[5,l-c][l,2,4]benzotπazme, l,8-Bis-(2-fluoro-5-propoxy-phenyl)-3-methyl-imidazo[5,l- c] [1 ,2,4]benzotnazme, ljS-Bis-CS-butoxy^-fluoro-pheny^-S-methyl-imidazofS.l-cJfl^^Jbenzotπazme, l,8-Bis-(2-fluoro-5-tπfluoromethyl-phenyl)-3-methyl-imidazo[5,l- c][l ,2,4]benzotnazine, l,8-Bis-(2-fluoro-5-isoρroρoxy-phenyl)-3-methyl-imidazo[5,l- c] [ 1 ,2,4]benzotπazme,
[0267]1 ,8-Bis-(5-ethoxy-2-fluoro-phenyl)-3 -methyl -imidazo [5, 1 -c] [ 1 ,2,4]benzotπazme, l-Cyclohexyl-8-(2-cyclohexyl-4-methyl-imidazol-l-yl)-3-methyl-imidazo[5,l- c] [ 1 ,2,4]benzotnazme, l-(2,5-Dichloro-phenyl)-3-methyl-8-pipeπdm-l-yl-imidazo[5,l- c] [ 1 ,2,4]benzotπazine, l-(2,5-Dichloro-phenyl)-3-me(hyl-8-morpholm-4-yl-imidazo[5,l- c] [ 1 ,2,4]benzotnazine,
[0268]1 -Isobutyl-8-(2-isobutyl-4-methyl-imidazol- 1 -yl)-3 -methyl-imidazo[5, 1 - c] [ 1 ,2,4]benzotπazine, l-(2-Chloro-phenyl)-3-methyl-8-ρiρendm-l-yl-imidazo[5,l- c] [ 1 ,2,4]benzotnazine, l-(2-Chloro-phenyl)-3-methyl-8-morpholin-4-yl-imidazo[5,l- c][l,2,4]benzotnazme, i l-(2-Chloro-phenyl)-8-imidazol-l-yl-3-methyl-imidazo[5,l- c] [1 ,2,4]benzotnazine,
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT l-(2-Chloro-phenyl)-3-methyl-8-(4-memyl-piperazm-l-yl)-imidazo[5,l- c][l,2,4]benzotπazine, and l-(2-Chloro-phenyl)-8-(4-fluoro-benzyloxy)-3-methyl-imidazo[5,l- c] [ 1 ,2,4]benzotnazine, or a pharmaceutically acceptable salt thereof
[0269]The chemical compounds descπbed in this specification have been determined using either the ACDLABS 11 0 Name Pro Software (IUPAC Nomenclature of Organic Chemistry Rules, available from Advanced Chemistry Development, Inc ) or the ChemDraw Ultra 9 0 1 software (available from CambπdgeSoft) The following contains definitions of terms used in this specification The initial definition provided for a group or term herein applies to that group or term throughout the present specification, individually or as part of another group, unless otherwise indicated
[0270]At various places in the present specification, substituents of compounds of the invention are disclosed m groups or m ranges It is specifically intended that the invention include each and every individual subcombination of the members of such groups and ranges For example, the term "Ci6 alkyl" is specifically intended to individually disclose methyl, ethyl, C3 alkyl, C4 alkyl, C5 alkyl, and Ce alkyl
[0271]It is further appreciated that certain features of the invention, which are, for clanty, described in the context of separate embodiments, can also be provided m combination in a single embodiment Conversely, vanous features of the invention which are, for brevity, described in the context of a single embodiment, can also be provided separately or in any suitable subcombination
[0272]The term "n-membered" where n is an integer typically descπbes the number of ring-forming atoms in a moiety where the number of ring-forming atoms is n For example, pipeπdmyl is an example of a 6-membered heterocycloalkyl nng and 1,2,3,4- tetrahydro-naphthalene is an example of a 10-membered cycloalkyl group
[0273]For compounds of the invention m which a variable appears more than once, each variable can be a different moiety independently selected from the group defining the variable For example, where a structure is described having two R groups that are simultaneously present on the same compound, the two R groups can represent different
20743-0032WO1 (AM103S39; 44418; HUBR- 1344 PCT) PATENT moieties independently selected from the group defined for R In another example, when an optionally multiple substituent is designated in the form
 then it is understood that substituent R can occur p number of times on the πng, and R can be a different moiety at each occurrence It is understood that each R group may replace any hydrogen atom attached to a πng atom, including one or both of the (CH2)n hydrogen atoms Further, in the above example, should the variable Q be defined to include hydrogens, such as when Q is said to be CH2, NH, etc , any floating substituent such as R in the above example, can replace a hydrogen of the Q vanable as well as a hydrogen m any other non- variable component of the πng
then it is understood that substituent R can occur p number of times on the πng, and R can be a different moiety at each occurrence It is understood that each R group may replace any hydrogen atom attached to a πng atom, including one or both of the (CH2)n hydrogen atoms Further, in the above example, should the variable Q be defined to include hydrogens, such as when Q is said to be CH2, NH, etc , any floating substituent such as R in the above example, can replace a hydrogen of the Q vanable as well as a hydrogen m any other non- variable component of the πng
[0274]For compounds of the invention in which a vanable appears more than once, each variable can be a different moiety independently selected from the group defining the vanable For example, where a structure is descnbed having two R groups that are simultaneously present on the same compound, the two R groups can represent different moieties independently selected from the group defined for R
[0275]As used herein, the phrase "optionally substituted" means unsubstituted or substituted As used herein, the term "substituted" means that a hydrogen atom is removed and replaced by a substitutent As used herein, the phrase "substituted with oxo" means that two hydrogen atoms are removed from a carbon atom and replaced by an oxygen bound by a double bond to the carbon atom It is understood that substitution at a given atom is limited by valency
[0276]The terms "alk" or "alkyl" refer to straight or branched chain hydrocarbon groups having 1 to 12 carbon atoms, preferably 1 to 8 carbon atoms or 1 to 6 carbon atoms The expression "lower alkyl" refers to alkyl groups of 1 to 4 carbon atoms The term "alkenyl" refers to straight or branched chain hydrocarbon groups of 2 to 10, preferably 2 to 4, or 2 to 6, carbon atoms having at least one double bond Where an alkenyl group is bonded to a nitrogen atom, it is preferred that such group not be bonded directly through a carbon beanng a double bond
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0277]The term "alkynyl" refers to straight or branched chain hydrocarbon groups of 2 to 10, preferably 2 to 4, or 2 to 6, carbon atoms having at least one triple bond Where an alkynyl group is bonded to a nitrogen atom, it is preferred that such group not be bonded directly through a carbon bearing a tnple bond The term "alkylene" refers to a straight chain bridge of 1 to 5 carbon atoms connected by single bonds (e g , -(CH2)X- wherein x is 1 to 5), which may be substituted with 1 to 3 lower alkyl groups
[0278]The term "alkenylene" refers to a straight chain bridge of 2 to 5 carbon atoms having one or two double bonds that is connected by single bonds and may be substituted with 1 to 3 lower alkyl groups Exemplary alkenylene groups are -CH=CH-CH-CH-, -CH2-CH=CH-, -CH2-CH=CH-CH2-,
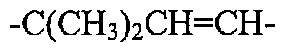 and -CH-(C2H5)-CH=CH-
and -CH-(C2H5)-CH=CH-
[0279]The term "alkynyl ene" refers to a straight chain bπdge of 2 to 5 carbon atoms that has a tnple bond therein, is connected by single bonds, and may be substituted with 1 to 3 lower alkyl groups Exemplary alkynylene groups are -O≡C-, -CH2-C=C-, -CH(CHa)- C≡C- and -C^C-CH(C2H5)CH2-
[0280]The terms "ar" or "aryl" refer to aromatic mono-, bi- or oligocyclic nngs, preferably phenyl, naphthyl and biphenyl In some embodiments, "ar" or "aryl" has 6 to 12 carbon atoms
[0281]As used herein, the term "alkylamino" refers to a group of formula -NH(alkyl), wherein the alkylene group and alkyl group each have 1 to 6 carbons
[0282]As used herein, the term "alkylcarbamyl" refers to a group of formula -C(O)- NH(alkyl), wherein the alkyl group has 1 to 6 carbons
[0283]As used herein, the term "alkyl carbamyloxy" refers to a group of formula -OC(O)NH(alkyl), wherein the alkyl group has 1 to 6 carbons As used herein, the term "alkoxy", employed alone or in combination with other terms, refers to an group of formula -O-alkyl Example alkoxy groups include methoxy, ethoxy, propoxy (e g , n-propoxy and isopropoxy), t-butoxy, and the like
[0284]As used herein, the term "alkoxycarbonyl" refers to a group of formula -C(O)O- alkyl As used herein, the term "alkylcarbonyl" refers to a group of formula -C(O)-alkyl
[0285]As used herein, the term "alkylsulfinyl" refers to a group of formula -S(O)-alkyl
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0286]As used herein, the term "alkylsulfonyl" refers to a group of formula -S(O)2- alkyl
[0287]As used herein, the term "alkylthio" refers to a group of formula -S-alkyl
[0288]As used herein, the term "ammo", employed alone or in combination with other terms, refers to a group of formula -NH2.
[0289]As used herein, the term "carbamyl" refers to a group of formula -C(O)NH2
[0290]As used herein, the term "carboxy" refers to a group of formula -C(O)OH
[0291]The terms "cycloalkyl" and "cycloalkenyl" refer to cyclic hydrocarbon groups of 3 to 8 carbon atoms hi some embodiments, one or more carbon atoms of the cycloalkyl or cycloalkenyl πng are oxidized to form a carbonyl group
[0292]As used herein, the term "dialkylammo" refers to a group of formula -N(alkyl)2, wherein the alkylene group and two alkyl groups each has, independently, 1 to 6 carbons
[0293]As used herein, the term "dialkylcarbamyl" refers to a group of formula -C(O)- N(alkyl)2, wherein the alkyl groups each has, independently, 1 to 6 carbons As used herein, the term "dialkylcarbamyloxy" refers to a group of formula
[0294]-OC(O)N(alkyl)2, wherein the alkyl groups each has, independently, 1 to 6 carbon atoms
[0295]As used herein, "haloalkoxy", employed alone or in combination with other terms, refers to a group of formula -O-haloalkyl An example haloalkoxy group is OCF3
[0296]As used herein, the term "haloalkyl", employed alone or in combination with other terms, refers to an alkyl group having from one halogen atom to 2n+l halogen atoms which may be the same or different, where "n" is the number of carbon atoms in the alkyl group
[0297]As used herein, the term "heterocycloalkyl" refers to a group of formula -alkyl- heterocyclo The terms "halogen" and "halo" refer to fluorine, chlorine, bromine and iodine
[0298]The term "unsaturated πng" includes partially unsaturated and aromatic πngs
[0299]The terms "heterocycle", "heterocyclic" or "heterocyclo" refer to fully saturated or unsaturated, including aromatic ("heteroaryl") or nonaromatic cyclic groups, for example, 4 to 7 membered monocyclic, 7 to 11 membered bicychc, or 10 to 15 membered tricyclic nng systems, which have at least one heteroatom in at least one carbon atom-containmg nng Each πng of the heterocyclic group containing a heteroatom
20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT may have 1, 2, 3 or 4 heteroatoms selected from nitrogen atoms, oxygen atoms and/or sulfur atoms, where the nitrogen and sulfur heteroatoms may optionally be oxidized and the nitrogen heteroatoms may optionally be quaternized The heterocyclic group may be attached at any heteroatom or carbon atom of the nng or πng system In some embodiments, one or more carbon atoms of the heterocyclo πng are oxidized to form a carbonyl group In some embodiments, the heterocyclo ring has 2 to 12, or 2 to 9 carbon atoms
[0300]Exemplary monocyclic heterocyclic groups include pyrrolidmyl, pyrrolyl, pyrazolyl, oxetanyl, pyrazolinyl, imidazolyl, lmidazolmyl, lmidazohdmyl, oxazolyl, oxazolidmyl, lsoxazolmyl, isoxazolyl, thiazolyl, tmadiazolyl, thiazolidmyl, isothiazolyl, isothiazolidmyl, furyl, tetrahydrofuryl, thienyl, oxadiazolyl, pipeπdmyl, piperazinyl, 2- oxopiperazmyl, 2-oxopipeπdmyl, 2-oxopyrrolodmyl, 2-oxoazepinyl, azepinyl, diazepinyl, 4-pipeπdonyl, pyπdyl, pyrazmyl, pynmidinyl, pyndazmyl, tetrahydropyranyl, morpholmyl, thiamorpholinyl, thiamorpholinyl sulfoxide, thiamorpholmyl, sulfone, 1,3- dioxolane and tetrahydro-1 , 1 -dioxothienyl, and the like
[0301]Exemplary bicyclic heterocyclic groups include indolyl, benzothiazolyl, benzoxazolyl, benzothienyl, qumuclidinyl, qumolinyl, tetra-hydroisoqumolmyl, isoqumolmyl, benzimidazolyl, benzopyranyl, mdohzmyl, benzofuryl, chromonyl, coumaπnyl, benzopyranyl, cinnolmyl, quinoxalmyl, indazolyl, pyrrolopyridyl, furopyπdmyl (such as furo[2,3-c]pyπdmyl, furo[3,2-b]pyndmyl or furo[2,3-b]pyπdmyl), dihydroisomdolyl, dihydroquinazolinyl (such as 3,4-dihydro-4-oxo-qumazolmyl), tetrahydroqumolmyl and the like
[0302]Exemplary tricyclic heterocyclic groups include carbazolyl, benzidolyl, phenanthrolmyl, acndinyl, phenanthπdmyl, xanthenyl and the like As used herein, the term "hydroxyl" refers to a group of formula -OH
[0303]As used herein, the term "mtro" refers to a group of formula -NO2
[0304]As used herein, the term "sulfinyl", employed alone or in combination with other terms, refers to -S(O)- group, which is a divalent one-sulfur moiety further bonded to an oxygen atom with a double bond
20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT
[0305]As used herein, the term "sulfonyl", employed alone or m combination with other terms, refers to a -S(O)2- group, which is a divalent one-sulfur moiety further bonded to two oxygen atoms via double bonds
[0306]As used herein, the term "thio", employed alone or in combination with other terms, refers to a -S- group, which is a divalent one-sulfur moiety
[0307]Throughout the definitions, the term "Cn m" is refered to indicate CM, CI ^, and the like, wherein n and m are integers and indicate the number of carbons, wherein n-m indicates a range which includes the endpomts
[0308]The compounds of formula I may form salts which are also withm the scope of this invention Reference to a compound of the formula I herein is understood to include reference to salts thereof, unless otherwise indicated The term "salt(s)", as employed herein, denotes acidic and/or basic salts formed with inorganic and/or organic acids and bases Zwitteπons (internal or inner salts) are included withm the term "salt(s)" as used herein (and may be formed, for example, where the R substituents compπse an acid moiety such as a carboxyl group) Also included herein are quaternary ammonium salts such as alkylammomum salts Salts of the compounds of the formula I may be formed, for example, by reacting a compound I with an amount of acid or base, such as an equivalent amount, m a medium such as one in which the salt precipitates or in an aqueous medium followed by lyophilization Exemplary acid addition salts include acetates (such as those formed with acetic acid or tπhaloacetic acid, for example, tnfluoroacetic acid), adipates, ahgmates, ascorbates, aspartates, benzoates, benzenesulfonates, bisulfates, borates, butyrates, citrates, camphorates, camphorsulfonates, cyclopentanepropionates, digluconates, dodecylsulfates, ethanesulfonates, fumarates, glucoheptanoates, glycerophosphates, hemisulfates, heptanoates, hexanoates, hydrochlorides, hydrobromides, hydroiodides, 2- hydroxyethanesulfonates, lactates, maleates, methanesulfonates, 2-naphthalenesulfonates, mcotmates, nitrates, oxalates, pectmates, persulfates, 3-phenylpropionates, phosphates, picrates, pivalates, propionates, salicylates, succinates, sulfates (such as those formed with sulfuric acid), sulfonates (such as those mentioned herein), tartrates, thiocyanates, toluenesulfonates such as tosylates, undecanoates, and the like
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0309]Exemplary basic salts (formed, for example, where the R substituents compπse an acidic moiety such as a carboxyl group) include ammonium salts, alkali metal salts such as sodium, lithium, and potassium salts, alkaline earth metal salts such as calcium and magnesium salts, salts with organic bases (for example, organic amines) such as benzathmes, dicyclohexylamines, hydrabamines, N-methyl-D-glucamines, N-methyl-D- glucamides, t-butyl amines, and salts with amino acids such as argimne, lysine and the like The basic nitrogen-containing groups maybe quatermzed with agents such as lower alkyl hahdes (e g , methyl, ethyl, propyl, and butyl chloπdes, bromides and iodides), dialkyl sulfates (e g dimethyl, diethyl, dibutyl, and diamyl sulfates), long chain halides (e g decyl, lauryl, myπstyl and stearyl chlorides, bromides and iodides), aralkyl halides (e g benzyl and phenethyl bromides), and others
[0310]The present invention also includes pharmaceutically acceptable salts of the compounds descnbed herein As used herein, "pharmaceutically acceptable salts" refers to denvatives of the disclosed compounds wherein the parent compound is modified by converting an existing acid or base moiety to its salt form Examples of pharmaceutically acceptable salts include, but are not limited to, mineral or organic acid salts of basic residues such as amines, alkali or organic salts of acidic residues such as carboxyhc acids, and the like The pharmaceutically acceptable salts of the present invention include the conventional non-toxic salts of the parent compound formed, for example, from non- toxic inorganic or organic acids The pharmaceutically acceptable salts of the present invention can be synthesized from the parent compound which contains a basic or acidic moiety by conventional chemical methods Generally, such salts can be prepared by reacting the free acid or base forms of these compounds with a stoichiometric amount of the appropπate base or acid m water or in an organic solvent, or in a mixture of the two, generally, nonaqueous media like ether, ethyl acetate, ethanol, isopropanol, or acetonitnle are preferred Lists of suitable salts are found in Remington 's Pharmaceutical Sciences, 17ft ed , Mack Publishing Company, Easton, Pa , 1985, p 1418 and Journal of Pharmaceutical Science, 66, 2 (1977), each of which is incorporated herein by reference in its entirety The phrase "pharmaceutically acceptable" is employed herein to refer to those compounds, materials, compositions, and/or dosage forms which are, withm the scope of
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT sound medical judgment, suitable for use in contact with the tissues of human bemgs and animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio
[0311]Furthermore, in the case of the compounds of the invention which contain an asymmetric carbon atom, the invention relates to the D form, the L form and D,L mixtures and also, where more than one asymmetric carbon atom is present, to the diastereomeπc forms Those compounds of the invention which contain asymmetric carbon atoms, and which as a rule accrue as racemates, can be separated mto the optically active isomers in a known manner, for example using an optically active acid However, it is also possible to use an optically active starting substance from the outset, with a corresponding optically active or diastereomeπc compound then being obtained as the end product
[0312]Compounds of the invention also include tautomeπc forms Tautomeric forms result from the swapping of a single bond with an adjacent double bond together with the concomitant migration of a proton Tautomenc forms include prototropic tautomers which are isomenc protonation states having the same empiπcal formula and total charge Example prototropic tautomers include ketone - enol pairs, amide - imidic acid pairs, lactam - lactim pairs, amide - imidic acid pairs, enamme — imine pairs, and annular forms where a proton can occupy two or more positions of a heterocyclic system, for example, IH- and 3H-imidazole, IH-, 2H- and 4H- 1 ,2,4-tπazole, IH- and 2H- lsomdole, and IH- and 2H-pyrazole Tautomeπc forms can be in eqmlibnum or steπcally locked into one form by appropriate substitution
[0313]The compounds descπbed herein can be asymmetπc (e g , having one or more stereocenters) All stereoisomers, such as enantiomers and diastereomers, are intended unless otherwise indicated Compounds of the present invention that contain asymmetncally substituted carbon atoms can be isolated in optically active or racemic forms Methods on how to prepare optically active forms from optically active starting mateπals are known in the art, such as by resolution of racemic mixtures or by stereoselective synthesis Many geometric isomers of olefins, C=N double bonds, and the like can also be present in the compounds descπbed herein, and all such stable isomers are contemplated in the present invention Cis and trans geometπc isomers of the
20743-0032WO1 (AM103539; 44418; HTIBR-1344 PCT) PATENT compounds of the present invention are described and may be isolated as a mixture of isomers or as separated isomenc forms
[0314]Compounds of the invention can also include all isotopes of atoms occurring in the intermediates or final compounds Isotopes include those atoms having the same atomic number but different mass numbers For example, isotopes of hydrogen include tritium and deuteπum
[0315]Also included are solvates and hydrates of the compounds of formula (I) and solvates and hydrates of their pharmaceutically acceptable salts
[0316]The term "compound" as used herein is meant to include all stereoisomers, geometric losomers, tautomers, and isotopes of the structures depicted, unless otherwise indicated
[0317]In some embodiments, the compound can be provided as a prodrug The term "prodrug", as employed herein, denotes a compound which, upon administration to a subject, undergoes chemical conversion by metabolic or chemical processes to yield a compound of the formula I, or a salt and/or solvate thereof
[0318]In some embodiments, the compounds of the invention, and salts thereof, are substantially isolated By "substantially isolated" is meant that the compound is at least partially or substantially separated from the environment m which it was formed or detected Partial separation can include, for example, a composition enriched m the compound of the invention Substantial separation can include compositions containing at least about 50%, at least about 60%, at least about 70%, at least about 80%, at least about 90%, at least about 95%, at least about 97%, or at least about 99% by weight of the compound of the invention, or salt thereof
[0319]Pharmaceutical Methods
[0320]The compounds according to the invention have been found to have pharmacologically important properties which can be used therapeutically The compounds of the invention can be used alone, in combination with each other or in combination with other active compounds Compounds of formula (I) may be inhibitors of phosphodiesterase 2 or 10 It is therefore a part of the subject-matter of this invention that the compounds of the invention and their salts and also pharmaceutical preparations
20743-0032WO1 (AM103S39, 44418; HUBR-1344 PCT) PATENT which compnse these compounds or their salts, can be used for treating or preventing disorders associated with, accompanied by and/or covered by phosphodiesterase hyperactivity and/or disorders in which inhibiting phosphodiesterase 2 or 10 is of value In some embodiments, the compound of formula I is selective for PDE 10, meaning that it is a better inhibitor of PDElO than for any other PDE In some embodiments, the selective PDElO inhibitor can reduce PDElO activity at least 10-fold or at least 100-fold compared to other PDE' s In some embodiments, the compound of formula I is a PDE2 selective inhibitor In some embodiments, the selective PDE2 inhibitor can reduce PDE2 activity at least 10-fold or at least 100-fold compared to other PDE's It is an embodiment of this invention, that compounds of the invention including their salts, solvates and hydrates, can be used for the treatment of central nervous system disorders of mammals including a human
[0321]More particularly, the mvention relates to the treatment of neurologic and psychiatric disorders including, but not limited to, (1) mood [affective] disorders, (2) neurotic, stress-related and somatoform disorders including anxiety disorders, (3) disorders compnsing the symptom of cognitive deficiency in a mammal, including a human, (4) disorders comprising attention deficits, executive function deficits (working memory deficits), dysfunction of impulse control, extrapyramidal symptoms, disorders that are based on a malfunction of basal ganglia, (5) behavioural and emotional disorders with onset usually occurring in childhood and adolescence, (6) disorders of psychological development, (7) systemic atrophies primarily affecting the central nervous system, (8) extrapyramidal and movement disorders, (9) behavioural syndromes associated with physiological disturbances and physical factors, (10) disorders of adult personality and behaviour, (11) schizophrenia and other psychotic disorders, (12) mental and behavioural disorders due to psychoactive substance use, (13) sexual dysfunction compnsing excessive sexual dπve, (14) mental retardation, (15) factitious disorders, (16) episodic and paroxysmal disorders, epilepsy, (17) narcolepsy, (18) dementia
[0322]Examples of mood [affective] disorders that can be treated according to the present invention include, but are not limited to, bipolar disorder I depressed, hypomamc, manic and mixed form, bipolar disorder II, depressive disorders, such as single depressive episode or recurrent major depressive disorder, minor depressive disorder,
20743-0032WO1 (AM103S39, 44418; HUBR- 1344 PCT) PATENT depressive disorder with postpartum onset, depressive disorders with psychotic symptoms, persistent mood [affective] disorders, such as cyclothymia, dysthymia, euthymia, and premenstrual dysphoric disorder
[0323]Examples of disorders belonging to the neurotic, stress-related and somatoform disorders that can be treated according to the present invention include, but are not limited to, anxiety disorders, general anxiety disorder, panic disorder with or without agoraphobia, specific phobia, social phobia, chrome anxiety disorders, obsessive compulsive disorder, reaction to sever stress and adjustment disorders, such as post traumatic stress disorder (PTSD), other neurotic disorders such as depersonalisation- derealisation syndrome
[0324]The phrase "cognitive deficiency" as used here in "disorder comprising as a symptom cognitive deficiency" refers to a subnormal functioning or a suboptimal functioning in one or more cognitive aspects such as memory, intellect, learning and logic ability, or attention and executive function (working memory) in a particular individual comparative to other individuals within the same general age population
[0325]Examples of disorders comprising as a symptom cognitive deficiency that can be treated according to the present invention include, but are not limited to cognitive deficits pπmaπly but not exclusively related to psychosis (schizophrenia), Parkinson's disease, Alzheimer's disease, multi infarct dementia, Lewis body dementia, stroke, frontotemporal dementia, progressive supranuclear palsy, Huntington's disease and in HIV disease, cerebral trauma and drug abuse, mild cognitive disorder and ADHD and Asperger's syndrome and age-associated memory impairment
[0326]Examples of disorders usually first diagnosed in infancy, childhood and adolescence that can be treated according to the present invention include, but are not limited to hyperkinetic disorders, including but not limited to disturbance of activity and attention, attention deficit/hyperactivity disorder (ADHD), hyperkinetic conduct disorder, attention deficit disorder (ADD), conduct disorders, including but not limited to depressive conduct disorder, tic disorders, including but not limited to transient tic disorder, chrome motor or vocal tic disorder, combined vocal and multiple motor tic disorder (de Ia Tourette), substance induced tic disorders, autistic disorders, excessive masturbation nail-biting, nose-pickmg and thumb-sucking
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0327]Examples of disorders of psychological development that can be treated according to the present invention include, but are not limited to pervasive developmental disorders, including but not limited to Asperger's syndrome and Rett's syndrome, autistic disorders, childhood autism and overactive disorder associated with mental retardation and stereotyped movements, specific developmental disorder of motor function, specific developmental disorders of scholastic skills
[0328]Examples of systemic atrophies primarily affecting the central nervous system that can be treated according to the present invention include, but are not limited to systemic atrophies primarily affecting the basal ganglia, including but not limited to Huntmgton's disease, multiple sclerosis, amyotrophic lateral sclerosis
[0329]Examples of movement disorders with malfunction and/or degeneration of basal ganglia that can be treated according to the present invention include, but are not limited to Parkinson's disease, second Parkinsonism, such as postencephalitic Parkinsonism, Parkinsonism compπsed in other disorders, Lewis body disease, degenerative diseases of the basal ganglia, other extrapyramidal and movement disorders including but not limited to tremor, essential tremor and drug-induced tremor, myoclonus, chorea and drug- induced chorea, drug-induced tics and tics of organic oπgion, drug-induced acute dystonia, drug-induced tardive dyskinesia, L-dopa-mduced dyskinesia, restless leg syndrome Stiff-man syndrome Further examples of movement disorders with malfunction and/or degeneration of basal ganglia that can be treated according to the present invention include, but are not limited to dystonia including but not limited to focal dystoma, multiple-focal or segmental dystoma, torsion dystoma, hemispheric, generalised and tardive dystoma (induced by psychopharmacological drugs) Focal dystoma include cervical dystoma (torticolh), blepharospasm (cramp of the eyelid), appendicular dystoma (cramp in the extremities, like the writer's cramp), oromandibular dystoma and spasmodic dysphoma (cramp of the vocal cord), neuroleptic-mduced movement disorders including but not limited to neuroleptic malignant syndrome (NMS), neuroleptic induced parkinsonism, neuroleptic-mduced early onset or acute dyskmesia, neuroleptic-induced acute dystoma, neuroleptic-mduced acute akathisia, neuroleptic-mduced tardive dyskinesia, neuroleptic- induced tremor
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT
[0330]Examples of behavioural syndromes associated with physiological disturbances and physical factors according to the present invention include, but are not limited to nonorganic sleep disorders, including but not limited to nonorganic hypersomnia, nonorganic disorder of the sleep-wake schedule, mental and behavioural disorders associated with the puerpeπum, including but not limited to postnatal and postpartum depression, eating disorders, including but not limited to anorexia nervosa and bulimia nervosa
[0331]Examples of disorders of adult personality and behaviour that can be treated according to the present invention include, but are not limited to personality disorders, including but not limited to emotionally unstable, borderline, obsessive-compulsive, anankastic, dependent and passive-aggressive personality disorder, habit and impulse disorders (impulse-control disorder), including intermittent explosive disorder, pathological gambling, pathological fire-settmg (pyromama), pathological stealing (kleptomania), trichotillomania, Munchausen syndrome Examples of schizophrenia and other psychotic disorders disorders that can be treated according to the present invention include, but are not limited to, continuous or episodic schizophrenia of different types (for instance paranoid, hebephrenic, catatonic, undifferentiated, residual, and schizophreniform disorders), schizotypal disorders (such as borderline, latent, prepsychotic, prodromal, pseudoneurotic pseudopsychopathic schizophrenia and schizotypal personality disorder), persistent delusional disorders, acute, transient and persistent psychotic disorders, induced delusional disorders, schizoaffective disorders of different type (for instance manic depressive or mixed type), puerperal psychosis and other and unspecified nonorganic psychosis
[0332]Examples of mental and behavioural disorders due to psychoactive substance use that can be treated according to the present invention include, but are not limited to mental and behavioural disorders due to use of alcohol, opioids, cannabmoids, sedatives or hypnotics, cocaine, mental and behavioural disorders due to the use of other stimulants, including caffeine, mental and behavioural disorders due to use of hallucinogens, tobacco, volatile solvents and mental and behavioural disorders due to multiple drug use and use of other psychoactive substances, including but not limited to
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT the following subtype symptoms harmful use, dependence syndrome, withdrawal state and withdrawal state with delirium
[0333]Examples of dementia that can be treated according to the present invention include, but are not limited to vascular dementia, dementia due to Creutzfeld-Jacob disease, HIV, head trauma, Parkinson's, Huntington's, Pick's disease, dementia of the Alzheimer's type
[0334]The compounds descnbed herein are further useful in the prevention and treatment of obesity, type 2 diabetes (non-msulm dependent diabetes), metabolic syndrome, glucose intolerance, and related health risks, symptoms or disorders As such, the compounds can also be used to reduce body fat or body weight of an overweight or obese individual
[0335]As used herein, the terms "overweight" and "obese" are meant to refer to adult persons 18 years or older having a greater than ideal body weight (or body fat) measured by the body mass index (BMI) BMI is calculated by weight m kilograms divided by height in meters squared (kg/m2) or, alternatively, by weight in pounds, multiplied by
[0336]703, divided by height in inches squared (lbs x 703/in2) Overweight individuals typically have a BMI of between 25 and 29, whereas obsess individuals typically have a BMI of 30 or more (see, e g , National Heart, Lung, and Blood institute, Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, The Evidence Report, Washington, DC U S Department of Health and Human Services, NIH publication no 98-4083,1998) Other means for indicating excess body weight, excess body fat, and obesity include direct measure of body fat and/or waist-to-hip ratio measurements
[0337]The term "metabolic syndrome" is used according to its usual meaning in the art The American Heart Association characterizes metabolic syndrome as having at least 3 of the 5 below symptoms 1) Elevated waist circumference (>102 cm (40 inches) in men, >88 cm (35 inches) in women), 2) Elevated tnglyceπdes (>150 mg/dL (>1 7 mmol/L) or drug treatment for elevated tnglyceπdes), 3) Reduced HDL-C (<40 mg/dL (1 03 mmol/L) in men <50 mg/dL (1 3 mmol/L) in women or drug treatment for reduced HDL- C, 4) Elevated blood pressure (>130/85 mmHg or drug treatment for hypertension), and 5) Elevated fasting glucose (>100 mg/dL or drug treatment for elevated glucose) See,
20743-0032WO1 (AM103S39; 44418; HUBR-1344 PCT) PATENT
[0338]Grundy, S M et al , Circulation, 2005, 112 (17, e285 (online at circ ahajournals org /cgi/repπnt/112/17/e285) Metabolic syndrome according to the World Health Organization (See, Alberti et al , Diabet Med 15, 539-553, 1998) includes individuals suffering from diabetes, glucose intolerance, low fasting glucose, or insulin resistance plus two or more of 1) High blood pressure (>160/90 mmHg), 2) Hyperlipdetma
[0339](triglycerides >150 mg/dL or HDL cholesterol <35 mg/dL in men and <39 mg/dL in women), 3) Central obesity (waist-to-hip ratio of >0 90 for men and >0 85 for women or BMI > 30 kg/m2), and 4) Microalbuminuria (urinary albumin excretion rate >20 μg/min or an albumm-to-creatine ratio >20 μg/kg) The compounds described herein are further useful in the prevention and treatment of disorders associated with enhanced endothelial activity, impaired endothelial barrier and/or enhanced neoangiogenesis, such as septic shock, vascular edema, reduced natπuna pathology, inflammatory diseases, including asthma, rhinitis, arthritis and rheumatoid diseases and autoimmune diseases, acute renal or liver failure, liver dysfunction, neoplasia benign and malignant
[0340]The compounds described herein are further useful m the prevention and treatment of disorders associated with thrombosis or embolism including, but not limited to thrombosis induced tissue infarction in coronary artery disease, in cerebrovascular disease and/or in peripheral vascular disease, stable and unstable angina, transient ischemic attacks, placenta insufficiency thrombosis after surgical procedures, such as bypass, angioplasty, stent placement, heart valve replacement
[0341]The present mvention also includes method of treating pain conditions and disorders Examples of such pain conditions and disorders include, but are not limited to, inflammatory pain, hyperalgesia, inflammatory hyperalgesia, migraine, cancer pain, osteoarthritis pain, post-surgical pain, non-inflammatory pain, neuropathic pain, sub- categories of neuropathic pain including peripheral neuropathic pain syndromes, chemotherapy-mduced neuropathy, complex regional pain syndrome, HIV sensory neuropathy, neuropathy secondary to tumor infiltration, painful diabetic neuropathy, phantom limb pain, postherpetic neuralgia, postmastectomy pain, bigeminal neuralgia, central neuropathic pain syndromes, central poststroke pain, multiple sclerosis pain, Parkinson disease pain, and spinal cord injury pain
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0342]As used herein, the term "treating" or "treatment" refers to one or more of (1) inhibiting the disease, for example, inhibiting a disease, condition or disorder in an individual who is expeπencing or displaying the pathology or symptomatology of the disease, condition or disorder (i e , arresting further development of the pathology and/or symptomatology), and (2) ameliorating the disease, for example, ameliorating a disease, condition or disorder m an individual who is expeπencing or displaying the pathology or symptomatology of the disease, condition or disorder (i e , reversing the pathology and/or symptomatology) such as decreasing the seventy of disease hi some embodiments, administration of a compound of the mvention, or pharmaceutically acceptable salt thereof, is effective in preventing the disease, for example, preventing a disease, condition or disorder in an individual who may be predisposed to the disease, condition or disorder but does not yet experience or display the pathology or symptomatology of the disease
[0343]Pharmaceutical Compositions
[0344]The present invention further provides pharmaceutical compositions comprising a compound of formula I or a pharmaceutically acceptable salt thereof In some embodiments, the composition further compπses a pharmaceutically acceptable earner An effective dose of the compounds according to the invention, or their salts, solvates or prodrugs thereof is used, in addition to physiologically acceptable earners, diluents and/or adjuvants for producing a pharmaceutical composition The dose of the active compounds can vary depending on the route of administration, the age and weight of the patient, the nature and seventy of the diseases to be treated, and similar factors The daily dose can be given as a single dose, which is to be administered once, or be subdivided into two or more daily doses, and is as a rule 0 001-2000 mg Particular preference is given to admimstenng daily doses of 0 1-500 mg, e g 0 1-100 mg
[0345]Suitable administration forms are oral, parenteral, intravenous, transdermal, topical, mhalative, intranasal and sublingual preparations Particular preference is given to using oral, parenteral, e g intravenous or intramuscular, intranasal preparations, e g dry powder or sublingual, of the compounds according to the mvention The customary galenic preparation forms, such as tablets, sugar-coated tablets, capsules, dispersible
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT powders, granulates, aqueous solutions, alcohol-containmg aqueous solutions, aqueous or oily suspensions, syrups, juices or drops, can be used
[0346]Solid medicinal forms can compπse inert components and carrier substances, such as calcium carbonate, calcium phosphate, sodium phosphate, lactose, starch, mannitol, alginates, gelatine, guar gum, magnesium stearate, aluminium stearate, methyl cellulose, talc, highly dispersed silicic acids, silicone oil, higher molecular weight fatty acids, (such as stearic acid), gelatine, agar agar or vegetable or ammal fats and oils, or solid high molecular weight polymers (such as polyethylene glycol), preparations which are suitable for oral administration can compπse additional flavourings and/or sweetening agents, if desired
[0347]Liquid medicinal forms can be sterilized and/or, where appropπate, compπse auxiliary substances, such as preservatives, stabilizers, wetting agents, penetrating agents, emulsifϊers, spreading agents, solubihzers, salts, sugars or sugar alcohols for regulating the osmotic pressure or for buffenng, and/or viscosity regulators Examples of such additives are tartrate and citrate buffers, ethanol and sequesteπng agents (such as ethylenediammetetraacetic acid and its non-toxic salts) High molecular weight polymers, such as liquid polyethylene oxides, microcrystalline celluloses, carboxymethyl celluloses, polyvinylpyrrolidones, dextrans or gelatine, are suitable for regulating the viscosity Examples of solid earner substances are starch, lactose, mannitol, methyl cellulose, talc, highly dispersed silicic acids, high molecular weight fatty acids (such as steanc acid), gelatine, agar agar, calcium phosphate, magnesium stearate, animal and vegetable fats, and solid high molecular weight polymers, such as polyethylene glycol
[0348]Oily suspensions for parenteral or topical applications can be vegetable, synthetic or semisynthetic oils, such as liquid fatty acid esters having in each case from 8 to 22 C atoms m the fatty acid chains, for example palmitic acid, launc acid, tndecanoic acid, margaπc acid, steanc acid, arachidic acid, myπstic acid, behemc acid, pentadecanoic acid, linoleic acid, elaidic acid, brasidic acid, erucic acid or oleic acid, which are esteπfied with monohydπc to tπhydnc alcohols having from 1 to 6 C atoms, such as methanol, ethanol, propanol, butanol, pentanol or their isomers, glycol or glycerol Examples of such fatty acid esters are commercially available miglyols, isopropyl
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT myπstate, isopropyl palmitate, isopropyl stearate, PEG 6-capnc acid, capryhc/capnc acid esters of saturated fatty alcohols, polyoxyethylene glycerol tnoleates, ethyl oleate, waxy fatty acid esters, such as artificial ducktail gland fat, coconut fatty acid isopropyl ester, oleyl oleate, decyl oleate, ethyl lactate, dibutyl phthalate, dnsopropyl adipate, polyol fatty acid esters, inter alia Silicone oils of differing viscosity, or fatty alcohols, such as isotndecyl alcohol, 2-octyldodecanol, cetylstearyl alcohol or oleyl alcohol, or fatty acids, such as oleic acid, are also suitable It is furthermore possible to use vegetable oils, such as castor oil, almond oil, olive oil, sesame oil, cotton seed oil, groundnut oil or soybean
[0349]Suitable solvents, gelatinizing agents and solubihzers are water or water-miscible solvents Examples of suitable substances are alcohols, such as ethanol or isopropyl alcohol, benzyl alcohol, 2-octyldodecanol, polyethylene glycols, phthalates, adipates, propylene glycol, glycerol, di- or tnpropylene glycol, waxes, methyl cellosolve, cellosolve, esters, morpholines, dioxane, dimethyl sulphoxide, dimethylformamide, tetrahydrofuran, cyclohexanone, etc
[0350]Cellulose ethers which can dissolve or swell both in water or in organic solvents, such as hydroxypropylmethyl cellulose, methyl cellulose or ethyl cellulose, or soluble starches, can be used as film-forming agents
[0351]Mixtures of gelatinizing agents and film-forming agents are also perfectly possible In this case, use is made, m particular, of iomc macromolecules such as sodium carboxymethyl cellulose, polyacrylic acid, polymethacrylic acid and their salts, sodium amylopectin semiglycolate, alginic acid or propylene glycol alginate as the sodium salt, gum arable, xanthan gum, guar gum or carrageenan The following can be used as additional formulation aids glycerol, paraffin of differing viscosity, tπethanolamine, collagen, allantoin and novantisolic acid Use of surfactants, emulsifiers or wetting agents, for example of Na lauryl sulphate, fatty alcohol ether sulphates, di-Na-N-lauryl-β- lmmodipropionate, polyethoxylated castor oil or sorbitan monooleate, sorbitan monostearate, polysorbates (e g Tween), cetyl alcohol, lecithin, glycerol monostearate, polyoxyethylene stearate, alkylphenol polyglycol ethers, cetyl tπmethyl ammonium chloride or mono-/dialkylρolyglycol ether orthophosphoric acid monoethanolamme salts can also be required for the formulation Stabilizers, such as montmonllomtes or colloidal
20743 0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT silicic acids, for stabilizing emulsions or preventing the breakdown of active substances such as antioxidants, for example tocopherols or butylhydroxyamsole, or preservatives, such as p-hydroxybenzoic acid esters, can likewise be used for preparing the desired formulations Preparations for parenteral administration can be present in separate dose unit forms, such as ampoules or vials Use is preferably made of solutions of the active compound, preferably aqueous solution and, in particular, isotonic solutions and also suspensions These injection forms can be made available as ready-to-use preparations or only be prepared directly before use, by mixing the active compound, for example the lyophihsate, where appropπate containing other solid earner substances, with the desired solvent or suspending agent
[0352]Intranasal preparations can be present as aqueous or oily solutions or as aqueous or oily suspensions They can also be present as lyophilisates which are prepared before use using the suitable solvent or suspending agent Inhalable preparations can present as powders, solutions or suspensions
[0353]Preferably, inhalable preparations are in the form of powders, e g as a mixture of the active ingredient with a suitable formulation aid such as lactose
[0354]The preparations are produced, ahquoted and sealed under the customary antimicrobial and aseptic conditions As indicated above, the compounds of the invention may be administered as a combination therapy with further active agents, e g therapeutically active compounds useful in the treatment of central nervous system disorders These further compounds may be PDE2 or PDEl 0 inhibitors or compounds which have an activity which is not based on PDE2 or PDElO inhibition such as NMDA modulating agents For a combination therapy, the active ingredients may be formulated as compositions containing several active ingredients in a single dose form and/or as kits containing individual active ingredients in separate dose forms The active ingredients used in combination therapy may be co-admmistered or administered separately
[0355]Synthesis
20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
[0356]Compounds of the invention, including salts thereof, can be prepared using known organic synthesis techniques and can be synthesized according to any of numerous possible synthetic routes
[0357]The reactions for preparing compounds of the invention can be earned out in suitable solvents which can be readily selected by one of skill in the art of organic synthesis Suitable solvents can be substantially non-reactive with the starting matenals (reactants), the intermediates, or products at the temperatures at which the reactions are earned out, e g , temperatures which can range from the solvent's freezing temperature to the solvent's boiling temperature A given reaction can be earned out in one solvent or a mixture of more than one solvent Depending on the particular reaction step, suitable solvents for a particular reaction step can be selected by the skilled artisan
[0358]Preparation of compounds of the invention can involve the protection and deprotection of various chemical groups The need for protection and deprotection, and the selection of appropnate protecting groups, can be readily determined by one skilled in the art The chemistry of protecting groups can be found, for example, m T W Greene and P G M Wuts, Protective Groups in Organic Synthesis, 3rd Ed , Wiley & Sons, Inc , New York (1999), which is incorporated herein by reference in its entirety
[0359]Reactions can be monitored according to any suitable method known in the art For example, product formation can be momtored by spectroscopic means, such as nuclear magnetic resonance spectroscopy (e g ,1H or13C), infrared spectroscopy, spectrophotometry (e g , UV-visible), mass spectrometry, or by chromatographic methods such as high performance liquid chromatography (HPLC) or thin layer chromatography (TLC)
[0360]Example synthetic methods for preparing compounds of the invention are provided in the Schemes below The compounds of the formula I may be prepared by methods such as those illustrated in the following Scheme 1
[0361]SS
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0363]
[0365]L = leaving group such as halo, tπflate, tosylate or mesylate
[0366]Scheme 1 shows that an appropriately substituted mtro benzene bearing a leaving group L (such as halo) 1 can be reacted with a substituted imidazole 2 in the presence of a base such as carbonates, hydroxides or an non-nucleophilic amine base The reaction may also be earned out in the presence of a Cu(I) salt Preferred leaving groups in 1 are F, Cl or Br The mtro group of 3 may then be reduced to provide the corresponding amine 4 by methods such as those known m the art, for example, by catalytic hydrogenation, by use of sodium dithiomte, SnCl2, or the like
[0367]The ammo group of 4 can then be reacted with a nitrite in the presence of an acid forming the corresponding diazonium salt which immediately forms the final product (I) by intramolecular coupling
[0368]In another approach to the compounds of the formula I (Scheme 2), a 2- haloimidazole as 5 can be utilized m the initial replacement of the leaving group on 1 to provide intermediates 6 This halo group can then be treated with aryl, heteroaryl boromc acids, boronate esters, or organotπfluoroborates (Suzuki coupling) to provide the
20743-0032WOl (AM103539; 44418; HUBR-1344 PCT) PATENT corresponding aryl or heteroaryl coupled products Ia In the event that imidazoles of type 7 are used in the displacement of the leaving group in 1, these can be converted to intermediates 9 after which the leaving group L2 can be installed, for example through brommation using N-bromosuccmimide The tnazmes 9 can then be transformed into the desired compounds of formula Ia The intermediates 6 can also undergo displacement with nucleophiles such as amines and alcohols (or thiols) in the presence of a base or under Cu(I) catalysis to provide compounds of formula Ib with a heteroatom containing group at R1
[0370]
[0372]R1 = aryl, heteroaryl
[0374]
[0375]Compounds of formula I can be prepared with various R groups through transformations on the benzene ring For instance, when R3 is a phenolic group, it can be
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT treated with hydrocarbyl halides, tosylates, mesylates and the like to transform the phenolic group to ethers. It should noted that in all of the Schemes described herein, if there are functional groups present on a substituent group such as R1, R2, R3 etc., further modification can be made if appropriate and desired. For example, a CN group can be hydrolyzed to afford an amide group; a carboxylic acid can be converted to a ester, which in turn can be reduced to an alcohol, which in turn can be further modified. In another example, an OH group can be converted into a better leaving group such as mesylate, which in turn is suitable for nucleophilic substitution, such as by CN. Furthermore, an OH group can be subjected to Mitsunobu reaction conditions with phenol, or hetereoaryl alcohol, to afford aryl or heteroaryl ether compounds. Although only few transformations are presented here, similar transformations are within the grasp of a skilled artisan.
[0376]In some embodiments, the present invention provides a method of preparing a compound of formula (I), comprising:
[0377](i) reacting an appropriately substituted nitro benzene of formula (1):
[0378]
[0379]1 with a substituted imidazole of formula (2):
[0382](ii) reducing the nitro group to an amino group;
[0383](iii) reacting the product of step (ii) with a nitrite in the presence of an acid to form the triazine ring structure; wherein L is a leaving group.
[0384]In some embodiments, step (i) is accomplished in the presence of a base.
20743-0032WO1 (AM103S39; 44418; HUBR-1344 PCT) PATENT
[0385]In some embodiments, the base is selected from a carbonate, hydroxide and amine base
[0386]In some embodiments, L is selected from fluoro, chloro, and bromo In some embodiments, the mtro group in (11) is reduced by catalytic hydrogenation, by use of sodium dithionite, or by use of SnCU
[0387]In some embodiments, the acid in (in) is selected from a mineral acid In some embodiments, the acid m (m) is selected from HCl and H2SO4 The invention will be described in greater detail by way of specific examples The following examples are offered for illustrative purposes, and are not intended to limit the invention m any manner Those of skill in the art will readily recognize a vanety of non- critical parameters which can be changed or modified to yield essentially the same results
[0389]Example 1: 8-fluoro-3-methyl-l-propyl-imidazo[5,l-c][l,2,4]benzotriazine
[0390] Step 1 4-fluoro-2-(4-methyl-2-propyl-ιmιdazol-l-yl)-nιtrobenzene
Step 1 4-fluoro-2-(4-methyl-2-propyl-ιmιdazol-l-yl)-nιtrobenzene
[0391]
[0392]To a suspension OfK2CO3 (15 g), 4-methyl-2-propyl imidazole (from Ferak Berlin GmbH, Germany, 6 5 g) and 100 ml acetomtnle was added 2,4-difluoro- mtrobenzene (from ABCR GmbH & Co KG Karlsruhe, Germany or Sigma-Aldπch Co , 8 g) The reaction mixture was stirred and heated to reflux for 7 h Then the reaction mixture was filtered off The solvent was removed and the crude residue was purified by chromatography (dichloromethane (DCM)Λutanol 97 3) Yield 72 g, m p 36-390C
20743-0032WO1 (AM103539; 44418, HϋBR-1344 PCT) PATENT
[0393]Step 2 l-amιno-4-fluoro-2-(4-methyl-2-propyl-ιmιdazol-l-yl)-benzene
[0394]
[0395]To a solution of 4-fluoro-2-(4-methyl-2-propyl-imidazol-l-yl)-mtrobenzene (2 8 g) and 100 ml ethanol, was added palladium-charcoal (1 g) The reaction mixture was heated to 400C and then hydrogenated under pressure (10 to 15 bar) The catalyst was filtrated off at room temperature (RT) and the filtrate was evaporated To the solid residue methyl tert-butyl ether (MTBE, 20 ml) was added After stirring for 30 minutes the product was collected by filtration, washed with 5 ml MTBE for 2 times and dried in a dry box with vacuum (400C) Yield 1 O g, m p 38-420C
[0396]Step 3 8-fluoro-3-methyl-l-propyl-ιmιdazo[5,l-c][l,24]benzotrιazιne
[0397] l-amino-4-fluoro-2-(4-methyl-2-propyl-imidazol-l-yl)-benzene (2 g) was dissolved in 25 ml of 1 M H2SO4 This solution was stirred and cooled with ice A solution of sodium nitrite (1 g) m 10 ml water was added to the mixture over a peπod of time of 30 minutes It was stirred for additional 2 h at about 00C A crude yellow product precipitated It was filtered off and re-crystallized from iso-propanol Yield 3 6 g, m p 124 5-126 50C, MS [M+H]+ 245
l-amino-4-fluoro-2-(4-methyl-2-propyl-imidazol-l-yl)-benzene (2 g) was dissolved in 25 ml of 1 M H2SO4 This solution was stirred and cooled with ice A solution of sodium nitrite (1 g) m 10 ml water was added to the mixture over a peπod of time of 30 minutes It was stirred for additional 2 h at about 00C A crude yellow product precipitated It was filtered off and re-crystallized from iso-propanol Yield 3 6 g, m p 124 5-126 50C, MS [M+H]+ 245
[0398]Example 2: 7-methoxy-3-methyl-l-propyl-imidazo[S,l-c][l,2,4]benzotriazine.
[0399] 20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
[0400]This compound was prepared as descπbed in Example 1 by replacing 2,4- difluoro-mtrobenzene with l-fluoro-4-methoxy-2-mtrobenzene m step 1 m p 159-1620C, MS [M+H]+ 257
[0401]Example 3: l-ethyl-8-fluoro-3-methyl-imidazo[5,l-c][l,2,4]benzotriazine.

[0402]This compound was prepared as descπbed m Example 1 by replacing 4-methyl-2- propyl imidazole with 4-methyl-2-ethyl imidazole (from ABCR GmbH & Co KG Karlsruhe, Germany) in step 1 m p 187-1900C, MS [M+H]+ 231
[0403]Example 4: l-cyclohexyl-S-fluoro-S-methyl-iπύdazo^l-cπi^^benzotriazine
[0404]Step 1 4-methyl-2-cyclohexyl imid
 6 5 g cyclohexyl aldehyde was stirred with 44 ml ethanol and 23 ml cone
6 5 g cyclohexyl aldehyde was stirred with 44 ml ethanol and 23 ml cone
[0405]NH3 H2O (32%) at RT for 30 minutes The mixture was heated to 50 to 600C 11 5 ml methyl glyoxal were added drop-wise The clear solution was stirred at 550C for 6 h At RT, 40 ml water were added The solvent ethanol was distilled off under reduced pressure The crude product precipitated It was filtered off washed with 2 x 30 ml water and dried at 300C Yield 7 7 g
[0406]Step 2 l-cyclohexyl-8-fluoro-3-methyl-ιmιdazo[5,l-c][l,2,4]benzotrιazιne
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0407]This compound was prepared as described in Example 1 by replacing 4-methyl-2- propyl imidazole with 4-methyl-2-cyclohexyl imidazole in step 1 m p 246-2500C (decomp ) , MS [M+H]+ 285
[0408]Example S: l-(2,5-dichlorophenyl)-8-methoxy-3-methyl-imidazo[5,l- c][l,2,4]benzotriazine.
[0409]
[0410]Step 1 2-(2,5-dιchlorophenyl)-4-methyl-lH-ιmιdazole
 10 0 g 2,5-dichlorobenzaldehyde was stirred with 44 ml ethanol and 23 ml cone
10 0 g 2,5-dichlorobenzaldehyde was stirred with 44 ml ethanol and 23 ml cone
[0411]NH3 H2O (32%) at RT for 30 minutes The mixture was heated to 50 to 60CC 11 5 ml methyl glyoxal were added drop-wise The clear solution was stirred at 550C for 6 h At RT, 40 ml water were added The solvent ethanol was distilled off under reduced pressure The crude product precipitated It was filtered off washed with 2 x 30 ml water and dried at 300C Yield 12 0 g
[0412]Step 2 l-(2,5-dιchlorophenyl)-8-fluoro-3-methyl-ιmιdazo[5,l-c][l,2,4]benzotnazιne
[0413]This compound was prepared as descπbed in Example 1 by replacing 2,4- difluoro-mtrobenzene with 2-fTuoro-4-methoxy- 1 -nitrobenzene and replacing 4-methyl-2- propyl imidazole with 4-(2,5-dichlorophenyl)-2-methyl-lH-imidazole m step 1 m p 165-1680C, MS [M+H]+ 359
[0414]20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
[0415]Example 6: l-(2,5-dichlorophenyl)-7-fluoro-3-methyI-imidazo[5,l- c] [l,2,4]benzotriazine.
[0416]
[0417]This compound was prepared as descπbed in Example 1 by replacing 2,4- difluoro-mtrobenzene with 1 ,4-difluoro-2-nitrobenzene (from ABCR GmbH & Co KG Karlsruhe, Germany) and replacing 4-methyl-2-propyl imidazole with 4-(2,5- dichlorophenyl)-2-methyl-lH-imidazole m step 1 m p 186-1900C, MS [M+H]+ 347
[0418]Example 7: l-(2,5-dichlorophenyl)-7-methoxy-3-methyl-imidazo[5,l- c] [1,2,4] benzotriazine.
[0419]
[0420]This compound was prepared as descπbed in Example 1 by replacing 2,4- difluoro-mtrobenzene with l-fluoro-4-methoxy-2-nitrobenzene and replacing 4-methyl-2- propyl imidazole with 4-(2,5-dichlorophenyl)-2-methyl-lH-imidazole in step 1 m p 144- 147 °C, MS [M+H]+ 359
[0421]The examples in Table 1 were prepared according to procedure descπbed for Example 1
[0422]Table 1
 20743-0032WO1 (AM103539, 44418, HUBR- 1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR- 1344 PCT) PATENT
[0423] 20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
[0424] 20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT

[0425]The examples in Table 2 were prepared according to procedure described for
[0428]
 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0429] 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT

[0430]The examples in Table 3 were prepared according to procedure described for
[0433]
 20743-0032WO1 (AM103S39; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103S39; 44418; HUBR-1344 PCT) PATENT
[0434]
[0435]Example 45: 6,8-dimethoxy-3-methyl-l-(4-methylpyridin-3-yl)imidazo[5,l- c] 11,2,4] benzotriazine
[0436]
[0437]Step 1 2-bromo-l-(3, 5-dιfluoro-2-nιtrophenyl)-4-methyl-lH-ιmιdazole
20743-0032WO1 (AM103S39, 44418, HTJBR-1344 PCT) PATENT
[0438]
[0439]A mixture of l,3,5-tπfluoro-2-mtrobenzene (2 2 mL, 18 6 ntmol), 2-bromo-4- methylimidazole (prepared according to EP 0514198, 3 g, 18 6 mmol) and K2CO3 (5 66 g, 41 mmol) in 80 mL DMF was stirred at RT overnight The mixture was diluted with ethyl acetate and washed with water Standard work up procedure followed by column purification using 10% ethyl acetate in dichloromethane as eluent provided 3 18 g (54% yield) of the product as a yellow powder EIMS 317 9 [M+H]+
[0440]Step 2 2-bromo-l-(3, 5-dιmethoxy-2-nιtrophenyl)-4-methyl-lH-ιmιdazole
[0441]
[0442]To a solution of 2-bromo-l-(3,5-difiuoro-2-mtrophenyl)-4-methyl-l.ff-imidazole (2 98 g, 9 4 mmol) in 40 mL MeOH was added freshly powdered KOH (2 5 g, 44 6 mmol) at RT under nitrogen The resulting mixture was stirred at 55 °C for 2 h, cooled to RT, diluted with dichloromethane and poured into water Standard work up and condensation on rotavap provided 3 35 g (100% yield) of the product as an off-white solid EIMS 342 0 [MH-H]+
[0443]Step 3 3-(l-(3,5-dιmethoxy-2-nιtrophenyl)-4-methyl-lH-ιmιdazol-2-yl)-4-methylpyrιdιne
[0444] To a mixture of 2-bromo-l-(3,5-dimethoxy-2-mtrophenyl)-4-methyl-liy- lmidazole (1 1 g, 3 2 mmol), 4-methyl-3-pyπdylboromc acid (commercially available
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT from Combi-Blocks Inc , 876 mg, 6 4 mmol), K2CO3 (1 32 g, 9 6 mmol) and Pd(PPh3)4 (186 mg, 0 161 mmol) in a 20 mL vial was added 60 mL of 1 ,4-dioxane and 20 mL of water under nitrogen The mixture was stirred at 900C for 4 h and cooled to RT Standard work up followed by column purification provided 788 mg (69% yield) of the desired product as an off-white powder EMS 355 1 [M+H]+
To a mixture of 2-bromo-l-(3,5-dimethoxy-2-mtrophenyl)-4-methyl-liy- lmidazole (1 1 g, 3 2 mmol), 4-methyl-3-pyπdylboromc acid (commercially available
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT from Combi-Blocks Inc , 876 mg, 6 4 mmol), K2CO3 (1 32 g, 9 6 mmol) and Pd(PPh3)4 (186 mg, 0 161 mmol) in a 20 mL vial was added 60 mL of 1 ,4-dioxane and 20 mL of water under nitrogen The mixture was stirred at 900C for 4 h and cooled to RT Standard work up followed by column purification provided 788 mg (69% yield) of the desired product as an off-white powder EMS 355 1 [M+H]+
[0445]Step 4 2, 4-dιmethoxy-6-(4-methyl-2-(4-methylpyrιdιn-3-yl)-lH-ιmιdazol-l-yl)anιlιne
[0446]
[0447]To a mixture of 3-(l-(3,5-dimethoxy-2-mtrophenyl)-4-methyl-lH-imidazol-2-yl)- 4-methylpyπdine (786 mg, 2 22 mmol) and 10% Pd/C (126 mg, 0 111 mmol) in a 100 mL RB flask (connected with a condenser) was added 10 mL THF, followed by slow addition of 10 mL MeOH with stirring HCOONH4 (832 mg, 12 21 mmol) was added in three portions into the stirring mixture and the final mixture was stirred at RT for 10 mm (gas released) and then warmed to 500C for 1 h The reaction was cooled to RT and filtered through Cehte Solvent was evaporated by rotovap and the residue was partitioned between water (~50 mL) and ethyl acetate (~80 mL) Aqueous phase was extracted with ethyl acetate (3 x 50 mL) The combined organic phase was dπed over MgSO4 Condensation followed by column purification using 10% methanol in dichloromethane as eluent provided 460 mg (64% yield) of the product as an off-white powder EIMS 325 1 [M+H]+
[0448]Step 5 6, 8-dιmethoxy-3-methyl-l-(4-methylpyrιdιn-3-yl)ιmιdazo[5,l- c][l 2,4]benzotnazme
[0449]To a solution of 2,4-dimethoxy-6-(4-methyl-2-(4-methylpyπdm-3-yl)-lH- imidazol-l-yl)amline (102 mg, 0 314 mmol) in 4 mL of AcOH was added a solution of NaNO2 (33 mg, 0 48 mol) in 0 6 mL of water at RT The resulting mixture was stirred at
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0450]RT for 1 h and quenched with NaHCU3 solution (make sure pH > 7) Extraction with ethyl acetate and condensation on rotavap provided the clean product (85 mg, 81% yield) as a yellow powder EIMS 336 1 [M+H]+
[0451]The examples in Table 4 were prepared according to procedure descπbed for Example 126
[0453]
[0454] 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0455] 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0456] 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0457]* Reduction performed using procedure from step 4 of Examples 67-98 **A = morpholinyl
[0459]The examples 67-98 were prepared according to procedure descnbed below and summarized in Table 5
[0460]
[0461]Step 1 3,5-dιfluoro-4-nιtrophenol [Al] and 3,5-dιfluoro-2-nιtrophenol [A2]
[0462]
[0463][A1] [A2] To a methylene chloride (150 mL) solution of 3,5-difluorophenol (14 08 g, 108 mmol) was added fummg nitric acid (>90%, 15 mL) in drop-wise (The addition speed significantly affects the ratio of A1/A2 — the slower the addition, the more product of Al) under N2 at 00C After addition, the resulting solution was stirred at the same temperature for 2 h The reaction was poured into cold water The organic layer was separated and the aqueous layer was extracted with methylene chloride Combined organic layer was washed with bπne and dried over magnesium sulfate Condensation under vacuo and column chromatography using 10-30% ethyl acetate in hexane provided 3,5-difluoro-4-nitrophenol [Al] as a thick yellow oil (5 1 g, 27% yield) and 3,5-difluoro- 2-nitrophenol [A2] as a yellow solid (8 0 g, 42% yield)
[0464]Step 2 l,3-dιfluoro-5-methoxy-2-nιtrobenzene
[0465] 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0466]To a mixture of 3,5-difluoro-4-mtrophenol (5 1 g, 29 mmol) and potassium carbonate (8 0 g, 58 mmol) in ΛζΛf-dimethylformamide (45 mL) was added MeI (22 mL, 34 8 mmol) at RT The resulting mixture was stirred at RT overnight Most solvent was removed under vacuo The residue was diluted with water and ethyl acetate Organic layer was separated and the aqueous layer was extracted with ethyl acetate Combined organic layer was washed with brine and dried over magnesium sulfate Condensation under vacuo and column chromatography using 20% ethyl acetate in hexane provided the product l,3-difluoro-5-methoxy-2-mtrobenzene (4 4 g, 81% yield)
[0467]Step 3 2-bromo-l-(3-fluoro-5-methoxy-2-nιtrophenyl)-4-methyl-lH-ιmιdazole
[0468]
[0469]To a mixture of l,3-difluoro-5-methoxy-2-mtrobenzene (9 g, 47 6 mmol) and potassium carbonate (14 5 g, 104 72 mmol) in 240 mL DMF was added 2-bromo-4- methyhmidazole (7 7 g, 47 6 mmol) at RT The resulting mixture was stirred at RT overnight Majority of solvent was removed by rotavap The residue was diluted with ethyl acetate and washed with water Extraction with ethyl acetate, condensation on rotavap, followed by column chromatography using 5-10% ethyl acetate m dichloromethane as eluent to provide 4 8 g (31%) titled product as a yellow solid
[0470]Step 4 2-(2-bromo-4-methyl-lH-ιmιdazol-l-yl)-4-methoxy-6-fluoroanιlιne
[0471]
[0472]A mixture of 2-bromo- 1 -(3-fluoro-5-methoxy-2-mtrophenyl)-4-methyl- IH- lmidazole (1 eq) and iron powder (6 eq) in a mixture solvent of AcOH and EtOH (1 1) was stirred at 100 °C under nitrogen for 3 h The hot mixture was filtered through Celite and washed (3X) with ethyl acetate Condensed on rotavap and the residue was
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT partitioned between sodium carbonate solution and ethyl acetate Extracted with ethyl acetate and purified by column to provide the desired product
[0473]Step 5 l-bromo-6-fluoro-8-methoxy-3-methylbenzo[e]ιmιdazo[5 l-c][l 2 4]tnazιne
[0474]
[0475]To a solution of 2-(2-bromo-4-methyl-lH-imidazol-l-yl)-4-methoxy-6- fluoroamlme (1 eq) in AcOH was added NaNC>2 (1 5 eq) in water (1 mL/1 mmol NaNθ2) solution at RT The resulting solution was stirred at RT for 1 h Worked up with sodium bicarbonate solution to make sure pH > 7 and extracted with ethyl acetate Purification by column provided l-bromo-6-fluoro-8-methoxy-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tπazme
[0476]Step 6 General Suzuki Coupling Preparation of compounds in Table 5
[0477] To a mixture of l-bromo-6-fluoro-8-methoxy-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tπazine (1 mmol), boronic acid (commercially available from Sigma-Aldnch Corporation, Boron Molecular Inc , SynQuest Laboratoπes, and Combi-Blocks Inc , 2 mmol), K2CO3 (3 mmol) and Pd(PPh3)4 (O 05 mmol) was added 20 mL of 1,4-dioxane and 7 mL of water under nitrogen The mixture was stirred at 90 °C for 4 h and cooled to RT Standard work up followed by column purification provided (70-80% yield) of the desired product
To a mixture of l-bromo-6-fluoro-8-methoxy-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tπazine (1 mmol), boronic acid (commercially available from Sigma-Aldnch Corporation, Boron Molecular Inc , SynQuest Laboratoπes, and Combi-Blocks Inc , 2 mmol), K2CO3 (3 mmol) and Pd(PPh3)4 (O 05 mmol) was added 20 mL of 1,4-dioxane and 7 mL of water under nitrogen The mixture was stirred at 90 °C for 4 h and cooled to RT Standard work up followed by column purification provided (70-80% yield) of the desired product
[0478]Table 5
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0479]
 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0480] 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT 18
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT 18
 20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT
[0481]
[0483]The examples in Table 6 were prepared according to procedure descπbed above for Examples 67-98 by replacing 3,5-difluoro-4-mtrophenol with 3,5-difluoro-2- nitrophenol (prepared as descπbed in step 1 of Examples 67-98) m Step 2
[0485]
 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0486] 20743-0032WO1 (AMI 03539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AMI 03539; 44418; HUBR-1344 PCT) PATENT
[0487]18

[0488]S3
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0489] 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0490]
[0491]Example 132: 6-Chloro-3-methyl-l-(3-methylpyridin-4-yl)-8- (trifluoromethyl)imidazo[5,l- c] [l,2,4]benzotriazine
[0492]
[0493]Step 1 2-chloro-6-fluoro-4-(trιfluoromethyl)anιlιne
[0494]
[0495]2-Fluoro-4-(tπfluoromethyl)arulme (from Matnx Scientific, 5 g, 27 9 mmol) was dissolved in acetomtπle (100 mL) To this was added N-chlorosuccimimde (4 g, 30 7
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT mmol) The reaction was heated to 750C for 16 h then poured into water and extracted with ether The organic layer was separated and washed with saturated aqueous sodium bicarbonate then water, bπne and dπed over MgSθ4 The solution was filtered and the solvent removed under reduced pressure 5 2 g of yellow oil was recovered MS [(+)ESI] m/z = 212 8 [M-H]+
[0496]Step 2 l-Chloro-3-fluoro-2-mtro-5-(trιfluoromethyl)benzene
[0497] Sodium perborate tetrahydrate (7 3 g, 46 8 mmol) was suspended in glacial acetic acid (30 mL) and heated to 500C To this was added dropwise a solution of 2-chloro-6- fluoro-4-(tnfluoromethyl)amline (2 g, 9 37 mmol) dissolved m glacial acetic acid (20 mL) The reaction was stirred for 16 h at 500C The reaction was then poured into water and extracted with ether The organic layer was separated and washed with water, then dilute aqueous bicarbonate solution and bπne The organic layer was then dried over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in hexane 1 17 g of a brown oil was recovered MS [(+)ESI] m/z = 242 8 [M-H]+
Sodium perborate tetrahydrate (7 3 g, 46 8 mmol) was suspended in glacial acetic acid (30 mL) and heated to 500C To this was added dropwise a solution of 2-chloro-6- fluoro-4-(tnfluoromethyl)amline (2 g, 9 37 mmol) dissolved m glacial acetic acid (20 mL) The reaction was stirred for 16 h at 500C The reaction was then poured into water and extracted with ether The organic layer was separated and washed with water, then dilute aqueous bicarbonate solution and bπne The organic layer was then dried over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in hexane 1 17 g of a brown oil was recovered MS [(+)ESI] m/z = 242 8 [M-H]+
[0498]Step 3 l-(3-Chloro-2-nιtro-5-(trιfluoromethyl)phenyl)-4-methyl-lH-ιmιdazole
[0499] l-Chloro-3-fluoro-2-mtro-5-(tnfluoromethyl)benzene (1 1 g, 4 5 mmol) and 4- methyl-lH-irmdazole (from Sigma-Aldπch Co , 0 371 g, 4 5 mmol) were dissolved m DMF (IO mL) To this was added potassium carbonate (1 2 g, 9 0 mmol) The reaction was let stir at RT for 16 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water then bπne and dπed
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT over MgSθ4 The solution was then filtered and the solvent removed under reduced pressure The crude was purified by flash chromatography on silica gel m 10 2 hexane/ ethyl acetate 0 74 g of a tan solid was collected MS [(+)ESI] m/z = 306 0 [M-H]+
l-Chloro-3-fluoro-2-mtro-5-(tnfluoromethyl)benzene (1 1 g, 4 5 mmol) and 4- methyl-lH-irmdazole (from Sigma-Aldπch Co , 0 371 g, 4 5 mmol) were dissolved m DMF (IO mL) To this was added potassium carbonate (1 2 g, 9 0 mmol) The reaction was let stir at RT for 16 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water then bπne and dπed
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT over MgSθ4 The solution was then filtered and the solvent removed under reduced pressure The crude was purified by flash chromatography on silica gel m 10 2 hexane/ ethyl acetate 0 74 g of a tan solid was collected MS [(+)ESI] m/z = 306 0 [M-H]+
[0500]Step 4 2-Chloro-6-(4-methyl-lH-ιmιdazol-l-yl)-4-(tnfluoromethyl)anιlιne
[0501] l-(3-Chloro-2-nitro-5-(tπfluoromethyl)phenyl)-4-methyl-lH-imidazole (0 74 g, 2 4 mmol) was dissolved in a solution of glacial acetic acid and ethanol (10 mL each) To this was then added iron powder (081 g, 144 mmol) The reaction was heated to 1000C for 1 h The reaction was poured into aqueous sodium hydroxide IN and extracted with ethyl acetate The organic layer was separated then washed with water, bπne and dπed over MgSO4 The resulting solution was filtered and the solvent removed under reduced pressure An off white solid (0 68 g) was recovered MS [(+)ESI] m/z = 274 1 [M-H]+
l-(3-Chloro-2-nitro-5-(tπfluoromethyl)phenyl)-4-methyl-lH-imidazole (0 74 g, 2 4 mmol) was dissolved in a solution of glacial acetic acid and ethanol (10 mL each) To this was then added iron powder (081 g, 144 mmol) The reaction was heated to 1000C for 1 h The reaction was poured into aqueous sodium hydroxide IN and extracted with ethyl acetate The organic layer was separated then washed with water, bπne and dπed over MgSO4 The resulting solution was filtered and the solvent removed under reduced pressure An off white solid (0 68 g) was recovered MS [(+)ESI] m/z = 274 1 [M-H]+
[0502]Step 5 6-Chloro-3-methyl-8-(tnfluoromethyl)benzo[e]ιmιdazo[5,l-c][l,2,4]trιazιne
[0503]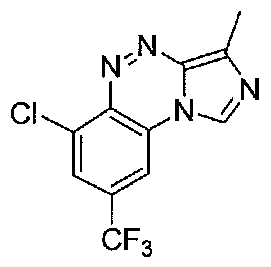
[0504]2-Chloro-6-(4-methyl-lH-imidazol-l-yl)-4-(tπfluoromethyl)amlme (l g, 3 6 mmol) was dissolved m glacial acetic acid (20 mL) To this was added sodium mtπte (025 g, 3 6 mmol)) The reaction was let stir 2 h at RT then poured into aqueous sodium hydroxide (IN) and extracted with ethyl acetate The organic layer was separated then washed with water, bπne and dπed over MgSθ4 The resulting solution was filtered and removed of solvent under reduced pressure The crude purified by flash chromatography
20743-0032WO1 (AM103S39; 44418; HUBR-1344 PCT) PATENT on silica gel in 10 2 hexane/ethyl acetate O 4 g of a yellow solid was collected MS [(+)ESI] m/z = 287 0 [M-H]+
[0505]Step 6 l-Bromo-6-chloro-3-methyl-8-(tnfluoromethyl)benzo[e]imidazo[5,l- c][l,2, 4]tnazme
[0506] δ-Chloro-S-methyl-δ^tnfluoromemytybeiizofelirnidazofSJ-cJfl^^triazme (045 g, 1 57 mmol) was dissolved in acetomtnle (20 mL) To this was added N- bromosuccmamide (1 1 g, 6 3 mmol)) The reaction was covered from light and stirred at RT for 48 h The reaction was poured into water and extracted with chloroform The organic layer was separated then bπne and dried over MgSO4 and filtered The solvent was removed under reduced pressure and the crude put on silica gel in 10 1 hexane/ethyl acetate 043 g of a yellow solid was recovered MS [(+)ESI] m/z = 364 9 [M-H]+
δ-Chloro-S-methyl-δ^tnfluoromemytybeiizofelirnidazofSJ-cJfl^^triazme (045 g, 1 57 mmol) was dissolved in acetomtnle (20 mL) To this was added N- bromosuccmamide (1 1 g, 6 3 mmol)) The reaction was covered from light and stirred at RT for 48 h The reaction was poured into water and extracted with chloroform The organic layer was separated then bπne and dried over MgSO4 and filtered The solvent was removed under reduced pressure and the crude put on silica gel in 10 1 hexane/ethyl acetate 043 g of a yellow solid was recovered MS [(+)ESI] m/z = 364 9 [M-H]+
[0507]Step 7 6-Chloro-3-methyl-l-(3-methylpyrιdιn-4-yl)-8-(trιfluoromethyl)ιmιdazo[5,l- c][l, 2, 4]benzotnazιne
[0508]l-Bromo-6-chloro-3-methyl-8-(tnfluoromethyl)benzo[e]imidazo[5,l- c][l,2,4]tnazme (0 1 g, 0 27 mmol) and 3-methylpyridm-4-ylboromc acid (from Asymchem Laboratories, Inc , 0 056 g, 0 41 mmol) were suspended in a solution containing dioxane (4 mL) and water (1 mL) To this was then added potassium carbonate (74 mg, 2 eq) Argon was bubbled thru the reaction for 1 mm and Pd(PPh3)4 (16 mg, 0 014 mmol) was added The reaction was sealed and heated to 110 °C for 3hrs The reaction was then poured into water and extracted with ethyl acetate The organic layer was bπne then dried over MgS O4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel m i l hexane/ethyl acetate (30 mg, 30% yield) of a yellow solid was collected MS [(+)ESI] m/z = 378 0 [M-H]+
20743-0032WO1 (AM103S39; 44418; HUBR- 1344 PCT) PATENT
[0509]Example 133: 6-chloro-l-(2,5-dichlorophenyl)-3-methyl-8- (trifluoromethyl)iraidazo[5,l-c][l,2,4]benzotriazine
[0510]Following the synthetic sequence m Example 132, the titled compound was prepared by replacing the 3-methylpyndm-4-ylboronic acid with 2,5- dichlorophenylboromc acid (from Sigma- Aldπch Co ) MS (ES) m/z 431 0 [M+H]+
[0511]Example 134 (Route 1): 6-methoxy-3-methyl-l-(3-methylpyridin-4-yl)-8- (trifluoromethyl)benzo[e]imidazo[S,l-c][l,2,4]triazine
[0512]
[0513]Step 1 l,3-dιfluoro-2-nιtro-5-(trιfluoromethyl)benzene
[0514]
[0515]Sodium perborate tetrahydrate (10 g, 4 eq) was suspended in glacial acetic acid (60 mL) and heated to 550C 2,6-difluoro-4-(tπfluoromethyl)aniline (prepared according to the procedure in European patent application EP315869 A2, 5 g, 25 4 mmol) dissolved m acetic acid (20 mL) was added drop-wise rapidly The reaction was stirred at 55 °C for 2 h The reaction mixture was poured into water and extracted with ether The organic layer was separated and washed with aqueous bicarbonate, water then separated, bπne and dπed over MgSC>4 and filtered The solvent was carefully removed (product volatile) under reduced pressure and the crude purified by flash chromatography on silica gel in hexane 0 198 g (35%, yield) of an orange oil was recovered MS [(+)ESI] m/z = 228 1 [M-H]+
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0516]Step 2 l-(3-fluoro-2-mtro-5-(trιfluoromethyl)phenyl)-4-methyl-lH-ιmιdazole
[0517]
[0518]4-methyl-lH-imidazole (2 433 g, 29 6 mmol) and l,3-difhioro-2-mtro-5- (tπfluoromethyl)benzene (6 73 g, 29 6 mmol) were dissolved in DMF (10 mL) To this was added K2CO3 (1 g, 2eq) The reaction was stirred at RT for 4 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water, brine and dπed over MgS O4 then filtered The solvent was removed under reduced pressure and the crude taken in small amount of CHCI3 and purified by flash chromatography on silica gel 20 to 30% hexane/ethyl acetate 42 g (49% yield) of a pale yellow solid was recovered MS [(+)ESI] m/z = 290 1 [M-H]+
[0519]Step 3 l-(3-methoxy-2-mtro-5-(trιfluoromethyl)phenyl)-4-methyl-lH-ιmιdazole
[0520] l-(3-fluoro-2-mtro-5-(tnfluoromethyl)phenyl)-4-methyl-lH-imidazole (02 g, 0 692 mmol) was dissolved in MeOH (3 mL) To this was added NaOMe/MeOH (25%) (2eq, 0 36 mL) The reaction darkened slightly and was stirred at RT for 2hrs The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water, bπne and dπed over MgSO4, then filtered The solvent was removed under reduced pressure 0 18 g ofa tan solid (90% yield) was recovered MS [(+)ESI] m/z = 302 1 [M-H]+
l-(3-fluoro-2-mtro-5-(tnfluoromethyl)phenyl)-4-methyl-lH-imidazole (02 g, 0 692 mmol) was dissolved in MeOH (3 mL) To this was added NaOMe/MeOH (25%) (2eq, 0 36 mL) The reaction darkened slightly and was stirred at RT for 2hrs The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water, bπne and dπed over MgSO4, then filtered The solvent was removed under reduced pressure 0 18 g ofa tan solid (90% yield) was recovered MS [(+)ESI] m/z = 302 1 [M-H]+
[0521]20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0522]Step 4 2-methoxy-6-(4-methyl-lH-imιdazol-l -yl)-4-(trιfluoromethyl)anιhne
[0523] l-(3-methoxy-2-mtro-5-(tπfluoromethyl)phenyl)-4-methyl-lH-imidazole (0 18 g, 0 598 mmol) was dissolved in a solution containing ethanol and glacial acetic acid (4 mL each) To this was added iron powder (0 2 g, 6 eq) The reaction was heated to 1000C for 1 h then poured into aqueous NaOH and extracted with ethyl acetate The organic layer was separated and washed with water, bπne and dπed over MgSO4, then filtered and removed of solvent under reduced pressure A tan solid 0 15 g (94% yield) was recovered MS [(+)ESI] m/z = 272 1 [M-H]+
l-(3-methoxy-2-mtro-5-(tπfluoromethyl)phenyl)-4-methyl-lH-imidazole (0 18 g, 0 598 mmol) was dissolved in a solution containing ethanol and glacial acetic acid (4 mL each) To this was added iron powder (0 2 g, 6 eq) The reaction was heated to 1000C for 1 h then poured into aqueous NaOH and extracted with ethyl acetate The organic layer was separated and washed with water, bπne and dπed over MgSO4, then filtered and removed of solvent under reduced pressure A tan solid 0 15 g (94% yield) was recovered MS [(+)ESI] m/z = 272 1 [M-H]+
[0524]Step 5 6-methoxy-3-methyl-8-(trιfluoromethyl)benzo[e]ιmιdazo[5, 1-c] [1,2,4] tnazine
[0525]
[0526]2-methoxy-6-(4-methyl-lH-imidazol-l-yl)-4-(tπfluoromethyl)anilme (0 15 g, 0 553 mmol) was dissolved in glacial acetic acid (4 mL) To this was added sodium nitrite (40 mg, 1 05 eq) The reaction turned yellow and was stirred to 1 h at RT then poured into aqueous NaOH and extracted with ethyl acetate The organic layer was separated and washed with water then bπne and dried over MgSO4 and filtered The solvent was removed under reduced pressure O H g (73 %) of a yellow solid was recovered MS [(+)ESI] m/z = 283 1 [M-H]+
[0527]20743-0032WO1 (AMΪ03539, 44418; HUBR- 1344 PCT) PATENT
[0528]Step 6 l-hromo-6-methoxy-3-methyl-8-(tnfluoromethyl)benzo[e]ιmιdazo[5, 1- c][l, 2, 4] tnazine
[0529]
[0530]6-methoxy-3 -methyl-8-(tnfluoromethyl)benzo [e]imidazo [5, 1 -c] [ 1 ,2,4]tπazine (0 1 g, 0 354 mmol) was dissolved m acetomtrile (5 mL) To this was added N-bromo succinamide (4 eq, 0 25 g) The reaction was protected from light and stirred at RT for
[0531]16 h A yellow solid formed and was filtered and washed with acetomtπle, then dπed
[0532]0 1 g (77% yield) of a yellow solid was recovered MS [(+)ESI] m/z = 361 0 [M-H]+
[0533]Step 7 6-methoxy-3-methyl-l-(3-methylpyndιn-4-yl)-8- (trιfluoromethyl)benzo[e]ιmιdazo[5, l-cjfl, 2, 4] tnazine
[0534]1 -bromo-6-methoxy-3-methyl-8-(tnfluoromethyl)benzo[e]imidazo[5, 1 - c][l,2,4]tnazme (0 1 g, 0277 mmol) and 3-methylpyπdm-4-ylboromc acid (0 076 g, 0 554 mmol) were suspended in a solution containing dioxane (4 mL) and water (1 mL) To this was added K2CO3 (2eq, 74 mg), argon was bubbled thru the reaction for 1 mm and Pd(PPh3 )4 (5% mol, 16 mg) was added The reaction was sealed and heated to 1100C for 16 h then diluted with water and extracted with ethyl acetate The organic layer was separated and dπed over MgSU4, filtered, and the solvent removed under reduced pressure The crude was purified by flash chromatography on silica gel in 10 2 methylene chloride/ ethyl acetate 30 mg (30% yield) of a yellow solid was recovered MS [(+)ESI] m/z = 374 1 [M-H]+ 1H NMR (400 MHz, d6-DMSO) δ ppm 8 76 (s, IH), 8 66 (d, J = 5 0 Hz, IH), 7 60 (d, J= 4 8 Hz, IH), 7 52 (s, IH), 6 75 (s, IH), 4 14 (s, 3H), 2 83 (s, 3H), 2 l l (s, 3H)
[0535]20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0536]Alternate Procedure for Example 134 (Route 2): 6-methoxy-3-methyl-l-(3- methylpyπdin-4-yl)-8-(trifluoromethyl)benzo[e]imidazo[5,l-c][l,2,4]triazine
[0537]
[0538]Step 1 2-bromo-l-(3-fluoro-2-nιtro-5-(trιfluoromethyl)phenyl)-4-methyl-lH-ιmιdazole
[0539] l,3-difluoro-2-mtro-5-(tπfluoromethyl)benzene (prepared as described above procedure, Step 1, 1 98 g, 8 72 mmol) and 2-bromo-4-methyl-lH-imidazole (1 404 g, 8 72 mmol) were dissolved in DMF (20 mL) To this was added K2CO3 (2 eq, 2 4 g) The reaction was stirred at RT for 1 5 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water then bπne, dried over MgSC>4 and filtered The solvent was removed under reduced pressure and the crude was taken in CHCI3 The resulting precipitate was filtered and collected The mother liquor was purified by flash chromatography on silica gel in 10 1 to 10 2 hexane/ethyl acetate 1 76 g (55% yield) of a pale yellow solid was recovered MS K+)ESI] m/z = 260 0 [M-H]+
l,3-difluoro-2-mtro-5-(tπfluoromethyl)benzene (prepared as described above procedure, Step 1, 1 98 g, 8 72 mmol) and 2-bromo-4-methyl-lH-imidazole (1 404 g, 8 72 mmol) were dissolved in DMF (20 mL) To this was added K2CO3 (2 eq, 2 4 g) The reaction was stirred at RT for 1 5 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water then bπne, dried over MgSC>4 and filtered The solvent was removed under reduced pressure and the crude was taken in CHCI3 The resulting precipitate was filtered and collected The mother liquor was purified by flash chromatography on silica gel in 10 1 to 10 2 hexane/ethyl acetate 1 76 g (55% yield) of a pale yellow solid was recovered MS K+)ESI] m/z = 260 0 [M-H]+
[0540]Step 2 2-bromo-l-(3-methoxy-2-nιtro-5-(tnfluoromethyl)phenyl)-4-methyl-lH- imidazole
[0541] 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0542]2-bromo-l-(3-fluoro-2-mtro-5-(tπfluoromethyl)phenyl)-4-methyl-lH-imidazole (1 3 g, 3 53 mmol) was dissolved in MeOH (15 mL) To this was added 25% NaOMe/MeOH (1 1 mL, 1 2 eq) The reaction was let stir at RT for lhr then poured into water and extracted with ethyl acetate The organic layer was separated and washed with water then brine and dried over MgSθ4, and filtered The solvent was removed under reduced pressure 1 25 g (93% yield) of a tan solid was recovered MS [(+)ESI] m/z = 380 0 [M-H]+
[0543]Step 3 4-(l-(3-methoxy-2-nitro-5-(tnfluoromethyl)phenyl)-4-methyl-lH-imidazol-2-yl)- 3-methylpyrιdιne
[0544]
[0545]2-bromo-l-(3-methoxy-2-mtro-5-(tnfluoromethyl)phenyl)-4-methyl-lH- lmidazole (1 25 g, 3 29 mmol) and 3-methylpyndm-4-ylboromc acid (0 901 g, 6 58 mmol) were dissolved in a solution containing dioxane (40 mL) and water (10 mL) To this was then added K2CO3 (2 eq, 0 9 g) and argon was bubbled through the reaction for 1 mm after which Pd(PPli3)4 (0 19 g, 5% mol) was added The reaction was sealed and heated to 110 °C for 7 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and bπne then dπed over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in 1 1 hexane/ethyl acetate 0 96 g (73% yield) of a pale yellow solid was recovered MS [(+)ESI] m/z = 393 1 [M-H]+
[0546]20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0547]Step 4 2-methoxy-6-(4-methyl-2-(3-methylpyndιn-4-yl)-lH-ιmιdazol-l-yl)-4- (tnfluoromethyl) aniline
[0548]
[0549]4-(l-(3-methoxy-2-mtro-5-(tnfluoromethyl)phenyl)-4-methyl-lH-imidazol-2-yl)- 3-methylpyπdme (0 9 g, 2 294 mmol) was dissolved m a solution of glacial acetic acid/ethanol (15 mL each) To this was added iron powder (0 7 g, 6 eq) The reaction was heated to 1000C for lhr The reaction was poured into dilute aqueous NaOH and extracted with ethyl acetate The organic layer was separated and washed with water then brme, dried over MgSθ4 and filtered The solvent was removed under reduced pressure 0 88 g (95% yields) of an off white solid was recovered MS [(+)ESI] m/z = 363 1 [M-H]+
[0550]Step 5 6-methoxy-3-methyl-l-(3-methylpyrιdιn-4-yl)-8- (trιfluoromethyl)benzo[e]ιmιdazo[5,l-c][l,2,4]tnazιne
[0551]2-methoxy-6-(4-methyl-2-(3-methylpyridin-4-yl)- 1 H-imidazol- 1 -yl)-4- (tnfluoromethyl)anilme (0 5 g, 1 380 mmol) was dissolved in glacial acetic acid (8 mL) To this was added sodium nitrite (0 1 g, 1 05 eq) The reaction turned dark clear yellow/ orange and was stirred for 0 5 h The reaction was poured into IN NaOH and extracted with CHCI3 The organic layer was separated and washed with water then bπne and dried over MgSθ4, filtered, and the solvent removed under reduced pressure The crude was purified by flash chromatography on silica gel in ethyl acetate 0 33g (66% yields) of a yellow solid was recovered MS [(+)ESI] m/z = 374 1 [M-H]+ 1H NMR (400 MHz, d6-DMSO) δ ppm 8 76 (s, IH), 8 66 (d, /= 5 0 Hz, IH), 7 60 (d, J= 4 8 Hz, IH), 7 52 (s, IH), 6 75 (s, IH), 4 14 (s, 3H), 2 83 (s, 3H), 2 11 (s, 3H)
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0552]Example 135: l-(2-Chlorophenyl)-6-methoxy-3-methyl-8- (trifluoromethyl)imidazo[5,l-c][l,2,4]benzotriazine
[0553]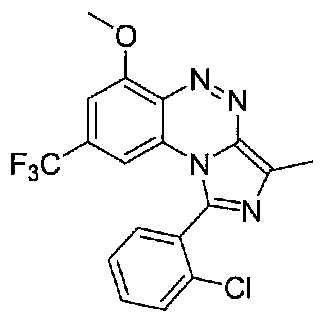
[0554]To a suspension of l-bromo-6-methoxy-3-meΛyl-8-(tafluoromethyl) benzo[e]imidazo[5,l-c][l,2,4]tπazme (prepared according to step 6 of Example 134, 0 100 g, 0277 mmol) in dioxane (4 mL) was added a solution of potassium carbonate (0 077 g, 2 eq) m water (1 mL) 2-Chlorophenyl boromc acid (from Sigma-Aldπch Co , 0065 g, 0415 mmol) was added , the mixture was degassed and Pd(PPh3)4 (0016 g) was added The mixture was once again degassed and heated to 1100C for 3 h (reaction monitored by LC/MS), then cooled to RT, diluted with water and extracted with ethyl acetate The combined extracts were dried over MgSθ4 and evaporated to dryness under reduced pressure The crude material was purified by flash chromatography (Isco CombiFlash Rf, 40 g Redi-Sep silica gel cartridge, gradient 5-80% EtOAc-hexane, 254 run detection) Pure fractions were combined and evaporated to dryness to provide the pure title compound as a yellow solid (0 100 g, 92 % yield) MS [(+)ESI, m/z] 393 1 [M+H]+ HRMS Calcd for Ci8H12ClF3N4O + H+, 393 0725 Found [(+)ESI, [M+H]+] 393 07321H NMR (400 MHz, DMSOd6) δ ppm 7 76 (m, 3H), 7 64 (m, IH), 7 52 (s, IH), 6 69 (s, IH), 4 13 (s, 3H), 2 84 (s, 3H)
[0555]Example 136: 6-Methoxy-3-methyl-l-(2-methylpyridin-3-yl)-8- (trifluoromethyl)imidazo[5,l-c][l,2,4]benzotriazine
[0556] 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0557]Step 1 2-Methyl-3-(4,4,5,5-tetramethyl-[l 3,2] dιoxaborolan-2-yl)-pyrιdιne
[0558]
[0559]3-Bromo-2-picolme (1 g, 5 8 mmol) was dissolved in dry DMF (20 mL) To this was added potassium acetate (2 g, 3 5 eq), followed by bis (pmacolato)diboron (1 9 g, 1 3 eq) Argon was bubbled through the reaction vessel for 1 mm and {1,1- bis(diphenylphosphino)ferrocene} palladium (II)-2CH2Cl2 (047 g, 10 mole%) was added The reaction was sealed and heated to 800C for 16 h, then cooled to room temperature and poured into water The aqueous solution was extracted with ethyl acetate and the organic layer separated, washed with bπne, dried over magnesium sulfate, filtered and the solvent was removed under reduced pressure The crude was purified by flash chromatography on silica gel in ethyl acetate 02 g of greenish oil was recovered, MS(ESI) 220 1 [M+l]+,1H NMR (400 MHz, ds-DMSO) δ ppm 8 45 (m,lH), 7 88 (m, IH), 7 14 (m, IH), 2 57 (s, 3H), 1 26 (s, 12H)
[0560]Step 2 6-Methoxy-3-methyl-l-(2-methylpyrιdιn-3-yl)-8-(trιfluoromethyl)ιmidazo[5,l- c][l 2,4]henzotrιazιne
[0561]Prepared according to the procedure of Example 135, using l-bromo-6-methoxy- 3-methyl-8-(tnfiuoromethyl) benzo[e]imidazo[5,l-c][l,2,4]triazme (0 100 g, 0 277 mmol), potassium carbonate (0 115 g, 3 eq), 2-methyl-3-(4,4,5,5-tetramethyl-l,3,2- dioxaborolan-2-yl)pyndme (0 121 g, 0 554 mmol) and Pd(PPh3)4 (0 016 g) in dioxane (4 mL) and water (1 mL) The mixture was stirred at 1100C for 5 h (monitored by LC/MS) Chromatography of the crude material (Isco, CombiFlash Rf, 40 g Redi-Sep silica gel cartridge, gradient 0-10% methanol in 60 40 dichloromethane- EtOAc, 254 nm detection) provided the pure title compound as a yellow solid (0 070 g, 68% yield) MS [(+)ESL m/z] 374 1 [M+H]+ HRMS Calcd for C18Hi4F3N5O + H+, 374 12232 Found [(+)ESI, [IVM-H]+], 374 12211H NMR (400 MHz, DMSO-d6) δ ppm 8 76 (m, IH), 8 0 (d, IH), 7 51 (s, IH), 7 48 (d, IH), 6 65 (s, IH), 4 13 (s, 3H), 2 83 (s, 3H), 2 24 (s, 3H)
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0562]Example 137: 6-Methoxy-3-methyI-l-(4-methylpyridin-3-yl)-8- (trifluoromethyl)imidazo[5,l-c][l,2,4]benzotriazine
[0563]
[0564]Prepared according to the procedure of Example 135, using l-bromo-6-methoxy- 3-methyl-8-(tnfluoromethyl) benzo[e]mudazo[5,l-c][l,2,4]tnazme (0 100 g, 0277 mmol), potassium carbonate (0 115 g, 3 eq), 4-methyl-pyridm-3-yl-boromc acid (from Combi-Blocks Inc , 0 076 g, 0 554 mmol) and Pd(PPh3)4 (0 016 g) in dioxane (4 mL) and water (1 mL) The mixture was stirred at 110 °C for 5 h (monitored by LC/MS), cooled, and additional boronic acid (1 equivalent) added along with Pd(PPh3)4 (0 010 g) Heating was resumed for additional 2 h at 110 °C Chromatography of the crude material (Isco, CombiFlash R6 40 g Redi-Sep silica gel cartridge, gradient of 0-40% EtOAc in dichloromethane followed by a gradient of 0-5% methanol in 60 40 dichloromethane- EtOAc, 254 nm detection) provided 0 079 g of a dark yellow solid The latter was dissolved in methanol, treated with activated charcoal, and filtered through a plug of Celite The cake was washed with methanol and ethyl acetate and the golden filtrate evaporated to dryness in vacuo to yield the pure title compound as a yellow solid (0 070 g, 68% yield)1H NMR (400 MHz, DMSO-d6) δ ppm 8 72 (d, IH), 8 69 (s, IH), 7 55 (d, IH), 7 51 (s, IH), 6 66 (s, IH), 4 13 (s, 3H), 2 84 (s, 3H), 2 11 (s, 3H)
[0565]Example 138: 6-Methoxy-3-methyl-l-(3-methyIthiophen-2-yl)-8- (trifluoromethyl)imidazo[5,l-c] [l,2,4]benzotriazine
[0566] 20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT
[0567]Prepared according to the procedure of Example 135, using 1 -bromo-6-methoxy- 3-methyl-8-(tπfluoromethyl) benzo[e]imidazo[5,l-c][l,2,4]tπazme (0 100 g, 0 277 mmol), potassium carbonate (0 115 g, 3 eq), 4)4,5,5-tetramethyl-2-(3-methylthiophen-2- yl)-l,3,2-dioxaborolane (from Sigma-Aldnch Co , 0 124 g, 0 554 mmol) and Pd(PPh3)4 (0 016 g) m dioxane (4 mL) and water (1 mL) The mixture was stirred at 1100C for 3 h (monitored by LC/MS) Chromatography of crude mateπal (Isco CombiFlash Rf, 40 g Redi-Sep silica gel cartridge, gradient 0-60% EtOAc-hexane, 254 ran detection) provided the pure title compound as a yellow solid (0 092 g, 88%) MS [(+)ESI, m/z] 379 1 [MH-H]+ HRMS Calcd for CnH13F3N4OS + H+, 379 0835 Found [(+)ESI, [MH-H]+, 379 08391H NMR (400 MHz, DMSO-d6) δ ppm 7 91 (d, IH), 7 52 (s, IH), 7 19 (d, IH), 6 99 (s, IH), 4 13 (s, 3H), 2 80 (s, 3H), 1 98 (s, 3H)
[0568]Example 139: 6-Methoxy-l-(3-methoxypyridin-4-yl)-3-methyl-8- (trifluoromethyl)benzo[e] imidazo[S,l-c] [l,2,4]triazine
[0569]
[0570]To a suspension of l-bromo-6-methoxy-3-methyl-8-(trifluoromethyl) benzo[e]imidazo[5,l-c][l,2,4]tnazme (prepared according to step 6 of Example 134, 0 110 g, 0 305mmol) in dioxane (6 mL) and 3 ml of water was added 5-methoxypyπdin- 3-ylboromc acid (from Combi-Blocks Inc , 0 058 g, 0 381 mmol), sodium carbonate (0 097 g, 0 914 mmol) and Pd(PPh3)4 (0 018 g, 0 015 mmol) in a sealed tube under N2 cover The reaction was heated to 1100C overnight then cooled to RT, diluted with water and extracted with ethyl acetate (2x) The combined extracts were dried over MgSO4 and evaporated to dryness under reduced pressure The crude mateπal was purified by flash chromatography (ISCO CombiFlash, 24 g Redi-Sep silica gel cartridge, gradient 5-80% EtOAc-DCM then MeOH-DCM gradient (5-30%) 0 05 g (42%) of the title product (yellow solid) was obtained MS ((+)ESI,m/z) 389 33(MH-H)+ 1H NMR
20743-0032WO1 (AM103539; 4441S, HUBR-1344 PCT) PATENT
[0571](400 MHz, CDCl3 ) δ ppm 8 55 (s, 2H),7 5(d, IH)J 3 (s, IH), 7 25 s, IH), 42 (s, 3H), 3 9(s, 3H), 2 95 (s, 3H)
[0572]Example 140: l-(2,5-Dichlorophenyl)-6-methoxy-3-methyI-8- (trifluoromethyl)benzo[e]imidazo[5,l-c] [1,2,4] triazine
[0573]
[0574]To a suspension of l-bromo-6-methoxy-3-methyl-8-(tnfluoromethyl) benzo[e]imidazo[5,l-c][l,2,4]tπazme (prepared according to step 6 of Example 134,0 13 g, 0 36mmol) in dioxane (6 mL) and 3 ml of water was added 2,5- dichloroophenylboronic acid (0086g, 0 45 mmol), sodium carbonate (0 114 g, 1 08 mmol) and Pd(PPh3)4 (0021 g, 0 018 mmol) in a sealed tube under N2 cover The reaction was heated to 1100C overnight, then cooled to RT, diluted with water and extracted with ethyl acetate (2x) The combined extracts were dπed over MgSO4 and evaporated to dryness under reduced pressure to provide O H g of crude brown solid The crude matenal was purified by flash chromatography (ISCO CombiFlash, 24 g Redi- Sep silica gel cartridge, gradient 5-80% EtOAc-DCM) 03 g (19 5%, yellow solid) of the title compound was recovered MS ((+)ESI,m/z) 427 21(M+H)+ 1H NMR (400 MHz, CDCl3) δ ppm 7 69(s,lH), 7 55 (m, 3H), 7 15(s,lH), 6 95 s IH)
[0575]Example 141: l-(3-Fluoro-2-methylphenyl)-6-methoxy-3-methyl-8- (trifluoromethyl)benzo[e] imidazo[S,l-c][l,2,4]triazine
[0576] 20743-0032WO1 (AM103S39, 44418, HϋBR-1344 PCT) PATENT
20743-0032WO1 (AM103S39, 44418, HϋBR-1344 PCT) PATENT
[0577]To a suspension of l-bromo-6-methoxy-3-methyl-8-(tπfluoromethyl) benzo[e]imidazo[5,l-c][l,2,4]tnazine (prepared according to step 6 of Example 134, 0 120 g, 0 332mmol) in dioxane (6 mL) and 3 ml of water was added 3-fluoro-2methyl- phenylboronic acid (from Combi-Blocks Inc , 0 064 g, 0415 mmol), sodium carbonate (0 106 g, 0 997 mmol) and Pd(PPh3)4 (0 019 g, 0 017 mmol) in a sealed tube under N2 cover The reaction was heated to 1100C overnight, then cooled to RT, diluted with water and extracted with ethyl acetate (2x) The combined extracts were dπed over MgSO4 and evaporated to dryness under reduced pressure The crude material was purified by flash chromatography (ISCO CombiFlash 24 g Redi-Sep silica gel cartridge, gradient 5-80% EtOAc-DCM) 0 06 g (50%) of the title compound was recovered MS ((+)ESI,m/z) 390 34 (M+H)+ 1H NMR (400 MHz, CDCl3) δ ppm 7 45 (m, IH), 7 35- 7 25 (m, 3H), 6 88(s,lH),4 19 (s,3H), 4 19 s,3H), 2 00 (s, 3H)
[0578]Example 142: l-(5-Chloro-2-methoxyphenyl)-6-methoxy-3-methyl-8- (trifluoromethyl)benzo[e] imidazo[5,l-c][l,2,4]triazine
[0579]
[0580]To a suspension of l-bromo-6-methoxy-3-methyl-8-(tnfluoromethyl) benzo[e]imidazo[5,l-c][l,2,4]tπazme (prepared according to step 6 of Example 134, 0 110 g, 0 305mmol) in dioxane (6 mL) and 3 ml of water was added 5-chloro-2- methoxy-phenylboromc acid (from Combi-Blocks Inc , 0071 g, 0 381mmol),sodmm carbonate (0 097 g, 0 914 mmol) and Pd(PPh3)4 (0 018 g, 0 015 mmol) in a sealed tube under N2 cover The reaction was heated to 110 °C for overnight then cooled to RT, diluted with water and extracted with ethyl acetate (2x) The combined extracts were dried over MgSO4 and evaporated to dryness under reduced pressure The crude mateπal was purified by flash chromatography (ISCO CombiFlash, 24 g Redi-Sep silica gel cartridge, gradient 10-80% EtOAc-hexane) 0 045 g (35%) of the title compound was recovered MS ((+)ESI,m/z) 423 1 (M+H)+ 1H NMR (400 MHz, CDCl3) δ ppm 7 68
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT
[0581](s, IH), 7 56 (d, IH), 7 25 (s,lH), 7 25 6 88 (d, IH), 3 58 (s, 3H), 2 94 (s, 3H), 2 92 (s, 3H)
[0582]Example 143: l-(Furan-3-yl)-6-methoxy-3-methyI-8- (trifluoromethyl)benzo[e]imidazo[5,l-c][l,2,4]triazine
[0583]
[0584]To a suspension of l-bromo-6-methoxy-3-methyl-8-(tπfluoromβthyl) benzo[e]imidazo[5,l-c][l,2,4]tπazine (prepared according to step 6 of Example 134, 0 120 g, 0 332 mmol) in dioxane (6 mL) and 3 ml of water was added furan-3-yl boromc acid (from Sigma- Aldπch Co , 0 046 g, 0 415 mmol), sodium carbonate (0 106 g, 0 997 mmol) and Pd(PPh3)4 (0 019 g, 0 017 mmol) in a sealed tube under N2 cover The reaction was heated to 1100C overnight then cooled to RT, diluted with water and extracted with ethyl acetate (2x) The combined extracts were dried over MgSθ4 and evaporated to dryness under reduced pressure The crude mateπal was purified by flash chromatography (ISCO CombiFlash, 24 g Redi-Sep silica gel cartridge, gradient 5-80% EtOAc-hexane) 0 075 g (65%) of the title compound was recovered as a yellow solid MS ((+)ESI,m/z) 348 28(M+H)+ 1H NMR (400 MHz, CDCl3) δ ppm 7 97(s,lH), 7 7 (s,lH), 7 68 (s,lH), 725 (d,lH) 4 19 s, 3H), 2 91 (s,3H)
[0585]Example 144: 4-(6-Methoxy-3-methyl-8-(trifluoromethyl)benzo[e]imidazo[5,l- c] [1,2,4] triazin-l-yl)-3,5-dimethylisoxazole
[0586] 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0587]To a suspension of l-bromo-6-methoxy-3-methyl-8-(tπfluoromethyl) benzo[e]tmidazo[5,l-c][l,2,4]tnazine (prepared according to step 6 of Example 134, 0 110 g, 0 305mmol) in dioxane (6 mL) and 3 ml of water was added 3,5- dimethylisoxazol-4-ylboronic acid (from Acros Orgamcs, 0048 g, 0 343 mmol), sodium carbonate (0 097 g, 0 914 mmol) and Pd(PPh3)4 (0 018 g, 0 015 mmol) in a sealed tube under N2 cover The reaction was heated to 1100C overnight then cooled to RT, diluted with water and extracted with ethyl acetate (2x) The combined extracts were dπed over MgSO4 filtered and stripped to a brown solid (0 13 g) The product was charged on an ISCO 24 g cartridge using a EtOAc DCM gradient 10-80% followed by a MeOH DCM (5-30%) gradient 0 060 g (52%) of the title compound was recovered as a tan solid MS ((+)ESI,m/z) 377 32 (M+H)+ 1H NMR (400 MHz, CDCl3) δ ppm 7 38 (s,lH),7 25(s,lH), 420 (s,3H), 2 93 (s,3H), 2 39 (s,3H), 2 17 (s, 3H)
[0588]Example 145: 6-Methoxy-3-methyl-l-(thiophen-2-yl)-8- (trifluoromethyl)benzo[e]imidazo [5,l-c][l,2,4]triazine
[0589]
[0590]To a suspension of l-bromo-6-methoxy-3-methyl-8-(tπfluoromethyl) benzo[e]imidazo[5,l-c][l,2,4]tπazme (prepared according to step 6 of Example 134, 0 110 g, 0 305 mmol) in dioxane (6 mL) and 3 ml of water was added thiophen-2-yl- boronic acid (from Sigma- Aldnch Co , 0 049 g, 0 381 mmol), sodium carbonate (0 097 g, 0 914 mmol) and Pd(PPh3)4 (0 015 g, 0 018mmol) m a sealed tube under N2 cover The reaction was heated to 1100C overnight then cooled to RT, diluted with water and extracted with ethyl acetate (2x) The combined extracts were dried over MgSO4 and evaporated to dryness under reduced pressure The crude material was purified by flash chromatography (ISCO CombiFlash 24 g Redi-Sep silica gel cartridge, gradient 5-80% EtOAc-DCM) 0 075 g (65%) of the title compound was recovered as a yellow solid
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0591]MS ((+)ESI,m/z) 364 34 (M+H)+ 1H NMR (400 MHz, CDCl3) δ ppm 7 7 (d,lH), 7 55(d,lH),748(d,lH), 726( d, IH), 7 21 (s,lH), 420(s,3H), 2 94(s,3H)
[0592]Example 146: l-(5-Fluoro-2-methylphenyl)-6-methoxy-3-methyl-8- (trifluoromethyl)benzo[e] imidazo[5,l-c] [l,2,4]triazine
[0593]
[0594]To a suspension of l-bromo-6-methoxy-3-methyl-8-(tπfluoromethyl) benzo[e]imidazo[5,l-c][l,2,4]tnazine (prepared according to step 6 of Example 134, 0 0 075 g, 0 208 mmol) in dioxane 6 mL and 3 ml water) was added 5-Fluoro-2- methylphenyl boronic acid (from Sigma-Aldπch Co , 0 040 g, 0 2601 mmol),sodmm carbonate (0 066 g, 0 625 mmol) and Pd(PPh3)4 (0 015 g, 0 018mmol) in a sealed tube under N2 cover The reaction was heated to 1100C for overnight then cooled to RT, diluted with water and extracted with ethyl acetate (2X) The combined extracts were dπed over MgSO4 and evaporated to dryness under reduced pressure The crude mateπal (0 10) was purified by flash chromatography (ISCO CombiFlash 12 g Redi-Sep silica gel cartridge, gradient 5-80% EtOAc-DCM 0 015g (18 5%) of the title compound was recovered as a yellow solid MS ((+)ESI,m/z) 390 33 (M+H)+ 1H NMR (400 MHz, CDCl3) δ ppm 7 40 (m,lH), 7 25 (m,lH), 7 21 (m, 2H), 6 89 (s,lH), 4 19(s,3H), 2 96 (s,3H), 2 02 (s,3H)
[0595]Example 149: 6-methoxy-3-methyI-l-(4-methylpyridin-3-yI)benzo[e]imidazo[5,l- c][l,2,4]triazin-8-ol
[0596] 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0597]Step 1 5-(benzyloxy)-l,3-dιfluoro-2-nιtrobenzene

[0598]To a mixture of intermediate Al (14 g, 80 mmol) and potassium carbonate (242 g, 176 mmol) in Λ^Λf-dimethylformamide (150 mL) was added benzyl bromide (1045 mL, 88 mmol) at RT The resulting mixture was stirred at RT overnight Most solvent was removed under vacuo The residue was diluted with water and ethyl acetate Organic layer was separated and the aqueous layer was extracted with ethyl acetate Combined organic layer was washed with brme and dned over magnesium sulfate Condensation under vacuo and column chromatography using 20% ethyl acetate m hexane provided the product (98% yield)
[0599]Step 2 l-(5-(benzyloxy)-3-fluoro-2-nitrophenyl)-2-bromo-4-methyl-lH-imidazole
[0600]
[0601]To a mixture of 5-(benzyloxy)-l,3-difluoro-2-nitrobenzene (2 65 g, 10 mmol) and potassium carbonate (2 76 g, 20 mmol) in 50 mL DMF was added 2-bromo-4- methyhmidazole (1 46 g, 9 09 mmol) at RT The resulting mixture was stirred at RT for 40 h Majority of solvent was removed by rotavap The residue was diluted with ethyl acetate and washed with water Extraction with ethyl acetate, condensation on rotavap, followed by column chromatography using 20-40% ethyl acetate in hexane as eluent to provide 1 72 g (47% yield) product as a thick foam
[0602]Step 3 l-(5-(benzyloxy)-3-methoxy-2-nιtrophenyl)-2-bromo-4-methyl-lH-ιmιdazole
[0603] 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0604]To a solution of l-(5-(benzyloxy)-3-fluoro-2-mtrophenyl)-2-bromo-4-methyl-lH- lmidazole (1 64 g, 4 04 mmol) in 20 rtiL of MeOH was added freshly powdered KOH (1 1 g, 194 mmol) at RT under nitrogen The resulting mixture was stirred at 550C for 2 h, then cooled to RT and diluted with water, extracted with ethyl acetate (3X) Standard work-up followed by column chromatography using 5-10% ethyl acetate in DCM as eluting solvent provided the product (1 65 g, 98% yield) as a yellow powder
[0605]Step 4 3-(l-(5-(benzyloxy)-3-methoxy-2-nιtrophenyl)-4-methyl-lH-ιmιdazol-2-yl)-4- methylpyndme
[0606]
[0607]Following the Suzuki coupling step 3 of Example 45, titled compound was prepared from l-(5-(benzyloxy)-3-methoxy-2-nitrophenyl)-2-bromo-4-methyl-lH- lmidazole in 82% yield EIMS 431 2 [M+l]+
[0608]Step 5 4-amιno-3-methoxy-5-(4-methyl-2-(4-methylpyrιdιn-3-yl)-lH-ιmιdazol-l- yl)phenol
[0609]
[0610]A mixture of 3-(l-(5-(benzyloxy)-3-methoxy-2-mtrophenyl)-4-methyl-lH- imidazol-2-yl)-4-methylpyndine (420 mg, 0 976 mmol) and palladium on carbon (104 mg, 0 098 mmol) was vacuumed and refilled with nitrogen, followed by addition of THF (10 ml) and MeOH (10 00 ml) The mixture was stirred at 600C for 2 h, cooled to RT, filtered through Celite, washed with ethyl acetate (3X) Evaporated to provide off-white
20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT solid 4-amino-3-methoxy-5-(4-methyl-2-(4-memylpyndm-3-yl)-lH-imidazol-l-yl)phenol (300 mg, 0 967 mmol, 99 %) EIMS 311 2 [M+l]+
[0611]Step 6 6-methoxy-3-methyl-l-(4-methylpyndιn-3-yl)benzo[e]ιmιdazo[5, 1- c][l,2,4]trιazιn-8-ol
[0612]The titled compound was prepared following step 5 of Example 45, from 4- ammo-3-methoxy-5-(4-methyl-2-(4-methylpyπdin-3-yl)-lH-imidazol-l-yl)phenol in 26% yield EIMS 322 2 [M+l]+
[0613]Example 150: 8-(difluoromethoxy)-6-methoxy-3-methyl-l-(4-methylpyridin-3- yl)benzo[e]imidazo[5,l-c][l,2,4]triaziαe
[0614]
[0615]A mixture of 6-methoxy-3-methyl-l-(4-methylpyπdin-3-yl)benzo[e]imidazo[5,l- c][l,2,4]tnazm-8-ol (Example 149, 70 mg, 0 218 mmol), cesium carbonate (355 mg,
[0616]1 089 mmol) and sodium 2-chloro-2,2-difluoroacetate (from TCI, Japan, 166 mg, 1 089 mmol) in a flask was vacuumed and refilled with nitrogen, followed by addition of DMF (5 4 ml) and water (0 600 ml) The resulting mixture was stirred at 1000C for 5 h Cooled to RT, diluted with water, extracted with ethyl acetate (3X) Column purification (50- 100%EtOAc in DCM, then 3-6%MeOH m DCM) provided the product 8-
[0617](difluoromethoxy)-6-methoxy-3-methyl- 1 -(4-methylpyndm-3-yl)benzo[e]imidazo[5, 1 - c][l,2,4]tπazme (E100519-35-1, 48 mg, 0 129 mmol, 59 3 %) EIMS 372 1 [MH-I]+ 1H NMR (400 MHz, d6-DMSO) δ ppm 8 70 (d, J = 5 1 Hz, IH), 8 64 (s, IH), 7 51 (d, J = 5 1 Hz, IH), 7 11 (t, J= 72 7 Hz, IH), 7 06 (d, J= 2 2 Hz, IH), 6 12 (d, /= 2 2 Hz, IH), 4 05 (s, 3H), 2 80 (s, 3H), 2 08 (s, 3H)
20743-0032WO1 (AM103539; 44418; HUBR- 1344 PCT) PATENT
[0618]Example 151 : 8-(benzyloxy)-6-methoxy-3-methyl-l-(3-methylpyridin-4- yl)imidazo[5,l- c] [l,2,4]benzotriazine
[0619]
[0620]Step 1 4-(l-(5-(benzyloxy)-3-methoxy-2-nιtrophenyl)-4-methyl-lH-ιmιdazol-2-yl)-3- methylpyridine
[0621]
[0622]The titled compound was prepared in 80% yield as descπbed in step 4 of Example 149 by replacing the 2-methylpyndin-3-ylborome acid with 3-methyIpyridm-4-ylboronic acid MS (ESI) 431 2 [M+l]+
[0623]Step 2 4-(benzyloxy)-2-methoxy-6-(4-methyl-2-(3-methylpyndιn-4-yl)-lH-ιmιdazol-l- yl)anιlιne
[0624] A mixture of 4-(l-(5-(benzyloxy)-3-methoxy-2-mtrophenyl)-4-methyl-lH- imidazol-2-yl)-3-methylpyπdine (412 mg, 0 957 mmol) and tin(II)chloπde dihydrate (1080 mg, 479 mmol) in a 250 mL flask was vacuumed and refilled with nitrogen, followed by the addition of ethanol (10 mL) and hydrochloric acid (37%, 0 286 mL)
20743-0032WO1 (AM103539; 44418; HUBR- 1344 PCT) PATENT
A mixture of 4-(l-(5-(benzyloxy)-3-methoxy-2-mtrophenyl)-4-methyl-lH- imidazol-2-yl)-3-methylpyπdine (412 mg, 0 957 mmol) and tin(II)chloπde dihydrate (1080 mg, 479 mmol) in a 250 mL flask was vacuumed and refilled with nitrogen, followed by the addition of ethanol (10 mL) and hydrochloric acid (37%, 0 286 mL)
20743-0032WO1 (AM103539; 44418; HUBR- 1344 PCT) PATENT
[0625]The final mixture was refluxed at 1000C for 2 h, cooled to RT The mixture was poured into cold IN NaOH solution, extracted with ethyl acetate (3X) Standard work-up followed by column chromatography (50-100% ethyl acetate in DCM, followed by 3- 8%MeOH in DCM) provided the product 4-(benzyloxy)-2-methoxy-6-(4-methyl-2-(3- methylpyπdin-4-yl)-lH-imidazol-l-yl)amlme (318 mg, 0 794 mmol, 83%) MS (ESI) 401 2 [M+l]+
[0626]Step 3 8-(benzyloxy)-6-methoxy-3-methyl-l-(3-methylpyndιn-4-yl)ιmιdazo[5,l- c][l, 2, 4]benzotnazιne
[0627]To a solution of 4-(benzyloxy)-2-methoxy-6-(4-methyl-2-(3-methylpyπdm-4-yl)- lH-imidazol-l-yl)amlme (318 mg, 0 794 mmol) in acetic acid (12 ml) was added a solution of sodium nitrite (82 mg, 1 191 mmol) in water (2 00 ml) at RT The resulting mixture was stirred at RT for Ih Solvent was removed by rotavap The residue was partitioned between ethyl acetate and sodium carbonate solution Extracted with ethyl acetate (3X) Washed with bπne Column purification (50-100% EtOAc in DCM, then 5- 10%MeOH in DCM) provided the product 8-(benzyloxy)-6-methoxy-3 -methyl- 1 -(3- methylpyπdin-4-yl)benzo[e]imidazo[5,l-c][l,2,4]tπazine (315 mg, 0 766 mmol, 96 %) as a yellow powder MS (ESI) 412 2 [M+l]+
[0628]Example 152: 6-methoxy-3-methyl-l-(3-methylpyridin-4-yl)benzo[e]imidazo[5,l- c][l,2,4]triazin-8-ol
[0629]
[0630]A mixture of 8-(benzyloxy)-6-methoxy-3-methyl-l-(3-methylpyπdm-4- yl)benzo[e]imidazo[5,l-c][l,2,4]tnazme (315 mg, 0 766 mmol) and palladium on carbon (40 7 mg, 0 038 mmol) in a flask was vacuumed and refilled with nitrogen, followed by addition of THF (8 ml) and MeOH (8 00 ml), then ammonium formate (145 mg, 2 297
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT mmol) was added in one portion The final mixture was stirred at 500C for 2 h Cooled to RT Filtered through Celite, extensively washed with ethyl acetate/MeOH until the filtrate becomes colorless Solvent was removed by rotavap The residue was purified by column (3-6-10% MeOH in DCM) provided the product 6-methoxy-3-methyl-l-(3- methylpyπdin-4-yl)benzo[e]imidazo[5,l-c][l,2,4]tπazin-8-ol (180 mg, 0 560 mmol, 73 2 %) MS (ESI) 322 1 [M+l]+
[0631]Example 153: 8-(difluoromethoxy)-6-methoxy-3-methyl-l-(3-methylpyridin-4- yl)imidazo[5,l- c] [l,2,4]benzotriazine
[0632]
[0633]A mixture of 6-methoxy-3-methyl- 1 -(3-methylpyndin-4-yl)benzo[e]imidazo[5! 1- c][l,2,4]tπazm-8-ol (80 mg, 0 249 mmol) and potassium carbonate (41 3 mg, 0299 mmol) in a vial was vacuumed and refilled with nitrogen, followed by addition of DMF (5 ml) and water (0 500 ml) The resulting mixture was warmed to 800C, then sodium 2- chloro-2,2-difluoroacetate (76 mg, 0498 mmol) was added quickly in one portion The final mixture was warmed to 1300C and stirred at this temperature for 2 h Cooled to RT Diluted with water and ethyl acetate Extracted with ethyl acetate (2X), washed with bπne, dried over magnesium sulfate Column purification (50-100%EtOAc in DCM) provided the product 8-(difluoromethoxy)-6-methoxy-3 -methyl- 1 -(3 -methylρyndin-4- yl)benzo[e]imidazo[5,l-c][l,2,4]tnazme (52 mg, 0 140 mmol, 56 2 %) as a yellow powder MS (ESI) 372 1 [M+lf1H NMR (400 MHz, d6-DMSO) δ ppm 8 70 (s, IH), 8 60 (d, J= 4 8 Hz, IH), 7 53 (d, J = 4 8 Hz, IH), 7 10 (t, J= 72 7 Hz, IH), 7 05 (d, J= 2 3 Hz, IH), 6 19 (d, J= 2 3 Hz, IH), 4 05 (s, 3H), 2 78 (s, 3H), 2 06 (s, 3H)
[0634]20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0635]Example 154: 8-(benzyloxy)-6-methoxy-3-methyI-l-(2-methylpyridin-3- yl)imidazo[5,l- c] [l,2,4]benzotriazine
[0636]
[0637]The titled compound was prepared following the procedure descπbed for Example 151 by replacing the 3-methylpyndm-4-ylboromc acid with 2-methylpyπdm-3- ylboromc acid MS (ESI) 412 2 [M+l]+
[0638]Example 155: 6-methoxy-3-methyl-l-(2-methylpyridin-3-yl)imidazo[5,l- c][l,2,4]benzotriazin-8- ol
[0639]
[0640]The titled compound was prepared following the procedure descnbed for Example 152 MS (ESI) 322 1 [M+l]+
[0641]Example 156: 8-(difluoromethoxy)-6-methoxy-3-methyl-l-(2-methylpyridin-3- yl)imidazo[5,l- c][l,2,4]benzotriazine
[0642]
[0643]The titled compound was prepared following the procedure descπbed for Example 153 MS (ESI) 372 1 [M+l]+ 1H NMR (400 MHz, ds-DMSO) δ ppm 8 71 (m,
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0644]IH), 7 92 (m, IH), 744 (m, IH), 7 09 (t, J= 72 7 Hz, IH), 7 04 (d, J= 2 3 Hz, IH), 6 10 (d, J= 2 3 Hz, IH), 4 05 (s, 3H), 2 78 (s, 3H), 2 22 (s, 3H)
[0645]Example 157: 8-(benzyloxy)-6-methoxy-3-methyl-l-(4-methylpyridin-3- yl)imidazo[S,l- c] [l,2,4]benzotriazine
[0646]
[0647]The titled compound was prepared following the procedure descπbed for Example 151 by replacing the 3-methylpyndm-4-ylboromc acid with 4-methylpyndm-3- ylboromc acid MS (ESI) 412 1 [M+l]+
[0648]Example 158: 8-(cyclopropylmethoxy)-6-methoxy-3-methyl-l-(3-methylpyridin-4- yl)benzo[e]imidazo[5,l-c] [1,2,4] triazine
[0649]
[0650]To a mixture of 6-methoxy-3-methyl-l-(3-methylpyπdm-4- yl)benzo[e]iimdazo[5,l-c][l,2,4]τπazm-8-ol (Example 152, 54 mg, 0 168 mmol) and cesium carbonate (82 mg, 0252 mmol) m 3 mL of DMF was added a solution of (bromomethyl)cyclopropane (from Matrix Scientific, 34 0 mg, 0 252 mmol) in 1 mL of DMF The resulting mixture was stirred at 100 °C overnight Cooled to RT Diluted with water and ethyl acetate Extracted with ethyl acetate (2X) Washed with bnne, dried over magnesium sulfate Column purification (50-100% EtOAc in DCM) provided the product (35 mg, 0 093 mmol, 55 5 %) as a yellow powder MS (ESI) 376 1 [M+l]+ IH NMR (400 MHz, d6-DMSO) δ ppm 8 70 (s, IH), 8 61 (d, J = 5 0 Hz, IH), 7 54 (d, J = 4 8 Hz,
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0651]IH), 6 78 (d, J = 2 3 Hz, IH), 5 90 (d, J = 2 3 Hz, IH), 4 01 (s, 3H), 3 50 (d, J = 7 0 Hz, 2H), 2 75 (s, 3H), 2 06 (s, 3H), 1 02 (m, IH), 0 51 (m, 2H), 0 18 (m, 2H)
[0652]Example 159: 8-(cyclopropylmethoxy)-6-methoxy-3-methyl-l-(2-methylpyridin-3- yl)benzo[e]imidazo[5,l-c][l,2,4]triazine
[0653]
[0654]To a mixture of 6-methoxy-3-methyl-l-(2-methylpyndin-3- yl)benzo[e]imidazo[5,l-c][l,2,4]tnazin-8-ol (Example 155, 30 mg, 0 093 mmol) and cesium carbonate (45 6 mg, 0 140 mmol) in 3 mL of DMF was added a solution of (bromomethyl) cyclopropane (18 91 mg, 0 140 mmol) in I mL of DMF The resulting mixture was stirred at 1000C overnight Cooled to RT Diluted with water and ethyl acetate Extracted with ethyl acetate (2X) Washed with bπne, dπed over magnesium sulfate Column purification (50-100% EtOAc in DCM) provided the product (28 mg,
[0655]0 075 mmol, 80 %) as a yellow powder MS (ESI) 376 1 [M+l]+ IH NMR (400 MHz, d6-DMSO) δ ppm 8 70 (m, IH), 7 95 (m, IH), 7 46 (m, IH), 6 77 (d, J = 2 3 Hz, IH),
[0656]5 84 (d, J = 2 3 Hz, IH), 4 00 (s, 3H), 3 48 (d, J = 7 0 Hz, 2H), 2 75 (s, 3H), 2 20 (s, 3H),
[0657]1 00 (m, IH), 0 51 (m, 2H), 020 (m, 2H)
[0659]
[0660]20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0661]Step 1 5-(dιfluoromethoxy)-l,3-dιfluoro-2-mtrobenzene

[0662]To a solution of 3,5-difluoro-4-mtrophenol (from step 1, Examples 67-98, 500 mg, 2 86 mmol) in acetomtnle (10 ml) was added a solution of potassium hydroxide (3365 mg, 60 0 mmol) in water (10 00 ml) To the resulting mixture was added 2-chloro- 2,2-difluoro-l-phenylethanone (from VWR, 2721 mg, 1428 mmol) at -780C The final mixture was warmed to RT, and then 800C overnight Cooled to RT diluted with water, extracted with ethyl acetate Column purification (5-10-20%EtOAc in Hexane) provided the product 5-(difluoromethoxy)-l,3-difluoro-2-mtrobenzene (280 mg, 1 244 mmol, 43 6 %) as a light brown oil MS (EI) 225 0 [M]+
[0663]Step 2 l-(5-(dιfluoromethoxy)-3-fluoro-2-nιtrophenyl)-4-methyl-lH-ιmιdazole
[0664]
[0665]A mixture of 5-(difluoromethoxy)-l,3-difluoro-2-nitrobenzene (2 g, 8 89 mmol), 4-methyl-lH-imidazole (0 729 g, 8 89 mmol) and potassium carbonate (2 456 g, 17 77 mmol) in DMF (80 ml) was stirred at 230C overnight Diluted with water and ethyl acetate Extracted with ethyl acetate (3X) Column purification (10-30% EtOAc in Hexane) provided the product l-(5-(difluoromethoxy)-3-fluoro-2-mtrophenyl)-4-methyl- lH-imidazole (1 7g, 66% yield) MS (ESI) 288 1 [M+l]+
[0666]Step 3 l-(5-(dιfluoromethoxy)-3-methoxy-2-nιtrophenyl)-4-methyl-lH-ιmιdazole
[0667] 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0668]To a solution of l-(5-(difluoromethoxy)-3-fluoro-2-mtrophenyl)-4-methyl-lH- lmidazole (1 7 g, 5 92 mmol) in MeOH (30 ml) was added freshly powdered potassium hydroxide (1 661 g, 29 6 mmol) the resulting mixture was stirred at 550C for 2 h Cooled to RT Solvent removed by rotavap, diluted with water and ethyl acetate, extracted with ethyl acetate (3X) Column purification (20-40% EtOAc in Hexane) provided the product l-(5-(dlfluoromethoxy)-3-methoxy-2-nitrophenyl)-4-methyl-lH- lmidazole (800 mg, 2 67 mmol, 45 2 %) MS (ESl) 300 1 [M+l]+
[0669]Step 4 4-(dιfluoromethoxy)-2-methoxy-6-(4-methyl-lH-ιmιdazol-l-yl)anιlιne
[0670]
[0671]To a mixture of l-(5-(difluoromethoxy)-3-methoxy-2-mtrophenyl)-4-methyl-lH- lmidazole (790 mg, 2 64 mmol) and palladium on carbon (140 mg, 0 132 mmol) was added THF (25 ml) and MeOH (25 00 ml) at RT under nitrogen, followed by addition of ammonium formate (499 mg, 7 92 mmol) The mixture was stirred at 500C for 1 h, cooled to RT filtered through Celite, washed with ethyl acetate Solvent was removed on rotavap to provide product (710 mg, 2 64 mmol, 100 %) as a pale oil MS (ESI) 270 1
[0672]Step 5 8-(difluoromethoxy)-6-methoxy-3-methylbenzo[e]imidazo[5,l-c][l,2,4]trwzine
[0673]
[0674]To a solution of 4-(difluoromethoxy)-2-methoxy-6-(4-methyl-lH-imidazol-l- yl)amline (710 mg, 2 64 mmol) m acetic acid (30 ml) was added a solution of sodium nitrite (280 mg, 4 06 mmol) in water (5 00 ml) The resulting mixture was stirred at RT for 2 h Solvent was removed by rotavap The residue was partitioned between sodium carbonate solution and ethyl acetate Organic phase was isolated and dried Column purification (50-100% EtOAc in DCM) provided the product The aqueous phase was
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT evaporated under vacuum to dryness MeOH was added to extract the product Short column purification (5-10%MeOH in DCM) provided the product (640 mg, 2 284 mmol, 87 %) as a yellow powder MS (ESI) 281 1 [M+l]+
[0675]Step 6 l-bromo-8-(dιfluoromethoxy)-6-methoxy-3-methylbenzo[e]ιmιdazo[5,l- c][l,2,4]tnazιne
[0676]
[0677]To a mixture of 8-(difluoromethoxy)-6-methoxy-3-methylbenzo[e]irmdazo[5,l- c][l,2,4]tπazme (592 mg, 2 113 mmol) and N-bromosuccimmide (564 mg, 3 17 mmol) in a flask was added acetomtπle (20 ml) under nitrogen at RT The resulting mixture was stirred at RT in darkness overnight Quenched with the reaction with sodium sulfite solution, extracted with ethyl acetate Column purification (30-50%EtOAc rn DCM) provided the product (700 mg, 1 949 mmol, 92 %) as a yellow powder MS (ESI) 359 0 [M+l]+
[0678]Step 7 General Suzuki Coupling Preparation of compounds in Table 7
[0679]A mixture of l-bromo-8-(difiuoromethoxy)-6-methoxy-3- methylbenzo[e]imidazo[5,l-c][l,2,4]triazine (60 mg, 0 167 mmol), various boronic acids (2 equivalents) or boronic ester (5 equivalents), potassium carbonate (69 3 mg, 0 501 mmol) and Pd(PPb^ (9 65 mg, 8 35 μmol) in a 40 mL vial was vacuumed and refilled with nitrogen, followed by addition of dioxane (4 5 ml) and water (1 500 ml) The resulting mixture was stirred at 900C overnight Cooled to RT Solvent was removed by rotavap The residue was purified by column to provide the product
[0680]The following compounds (Table 7) were prepared following the procedure described above
20743-0032WO1 (AM103S39; 44418; HUBR-1344 PCT) PATENT

 20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
[0681]
[0682]20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0683]
[0684]20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT
[0685]
[0686]Example 170: 8-(difluoromethoxy)-6-methoxy-l-(5-methoxypyridin-3-yI)-3- methylimidazo[5,l-c][l,2,4]benzotriazine
[0687]
[0688]Step 1 l-(5-fluoro-3-methoxy-2-nιtrophenyl)-4-methyl-lH-ιmιdazole
[0689] l,5-difluoro-3-methoxy-2-mtrobenzene (7 g, 37 0 mmol) and 4-methyl-lH- lmidazole (3 04 g, 37 0 mmol) were dissloved in DMF (100 mL). To this was added potassium carbonate (10 g, 74 mmol)) The reaction was stirred at RT overnight then poured into water and extracted with ethyl acetate The organic layer was separated, washed with water, bπne, then dπed over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
l,5-difluoro-3-methoxy-2-mtrobenzene (7 g, 37 0 mmol) and 4-methyl-lH- lmidazole (3 04 g, 37 0 mmol) were dissloved in DMF (100 mL). To this was added potassium carbonate (10 g, 74 mmol)) The reaction was stirred at RT overnight then poured into water and extracted with ethyl acetate The organic layer was separated, washed with water, bπne, then dπed over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0690]50% to 70% Ethyl acetate/ hexane 4 5 g of a pale yellow solid was recovered MS [(+)ESI] m/z = 252 1 [M-H]+
[0691]Step 2 2-bromo-l-(5-fluoro-3-methoxy-2-nιtrophenyl)-4-methyl-lH-ιmιdazole
[0692] l-(5-fluoro-3-methoxy-2-mtrophenyl)-4-methyl-lH-imidazole (4 9 g, 19 51 mmol) was dissolved m THF (40 mL) and cooled to 0 °C To this was added 1- bromopyrrolidine-2,5-dione (3 65 g, 2048 mmol) The reaction was let warm to RT after 1 h and stirred for 4 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and combined then bπne, dried over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in 20% to 40% ethyl acetate/hexane 1 6 g of a waxy yellow solid was collected MS [(+)ESI] m/z = 330 0 [M-H]+
l-(5-fluoro-3-methoxy-2-mtrophenyl)-4-methyl-lH-imidazole (4 9 g, 19 51 mmol) was dissolved m THF (40 mL) and cooled to 0 °C To this was added 1- bromopyrrolidine-2,5-dione (3 65 g, 2048 mmol) The reaction was let warm to RT after 1 h and stirred for 4 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and combined then bπne, dried over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in 20% to 40% ethyl acetate/hexane 1 6 g of a waxy yellow solid was collected MS [(+)ESI] m/z = 330 0 [M-H]+
[0693]Step 3 3-(2-bromo-4-methyl-lH-ιmιdazol-l-yl)-5-methoxy-4-nιtrophenol
[0694]
[0695]To 2-bromo-l-(5-fluoro-3-methoxy-2-mtrophenyl)-4-methyl-lH-imidazole (1 6 g, 4 85 mmol) was added 2-(methylsulfonyl)ethanol (0 903 g, 7 27 mmol) dissolved in DMF (10 mL), followed by powdered potassium hydroxide (0 81 g, 14 5 mmol ) The reaction was stirred for 1 h at RT then poured into saturated ammonium chloπde and extracted with ethyl acetate The organic layer was separated and washed with water then bπne , dπed over MgSθ4 and the solvent removed under reduced pressure, 1 6 g of a yellow solid was collected MS [(+)ESI] m/z = 328 0 [M-H]+
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0696]Step 4 2-bromo-l-(5-(dιfluoromethoxy)-3-methoxy-2-nιtrophenyl)-4-methyl-lH- imidazole
[0697]
[0698]3-(2-bromo-4-methyl-lH-imidazol-l-yl)-5-methoxy-4-mtrophenol (0 5 g, 1 524 5 mmol) and potassium carbonate (025 g, 0 18 mmol) were suspended in a solution containing DMF (6 niL) and water (0 6 mL) The reaction was heated to 80 °C and sodium 2-chloro-2,2-difluoroacetate (0 465 g, 3 05 mmol) was added m one portion The reaction was brought to 1100C and maintained for 1 h, then poured into saturated ammonium chloride and extracted with ethyl acetate The organic layer was separated o and washed with water then bπne and dried over MgS O4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in 20 to 50% ethyl acetate/ hexane 0 42 g of a tan solid was recovered MS [(+)ESI] m/z = 378 0 [M-H]+
[0699]5 Step 5 i-(l-(5-(dιfluoromethoxy)-3-methoxy-2-nιtrophenyl)-4-methyl-lH-ιmιdazol-2-yl)- 5-methoxypyrιdιne
[0700]
[0701]2-bromo-l-(5-(difluoromethoxy)-3-methoxy-2-mtrophenyl)-4-methyl-lH- lmidazole (0 15 g, 0 397 mmol) and 5-methoxypyπdin-3-ylboromc acid (0 121 g, 0 7930 mmol) were suspended in a solution containing dioxane (4 mL) and water (1 mL) To this was added potassium carbonate (162 mg, 1 2 mmol) Argon was bubbled through the reaction for 1 mm and Pd(PPh3 )4 (10% mol, 46 mg) was added The reaction was sealed and heated to 110 °C for 16 h The reaction was poured into water and extracted
20743-0032WO1 (AM103S39; 44418; HUBR-1344 PCT) PATENT with ethyl acetate The organic layer was separated and dπed over MgSC>4, filtered, and the solvent removed under reduced pressure The crude was purified by flash chromatography on silica gel m 50-100% ethyl acetate/hexane 0 15 g of a clear oil was recovered MS [(+)ESI] m/z = 407 1 [M-H]+
[0702]Step 6 4-(dιfluoromethoxy)-2-methoxy-6-(2-(5-methoxypyrιdιn-Z-yl)-4-methyl-lH- ιmιdazol-l-yl)anιhne
[0703]
[0704]3-(l-(5-(difluoromethoxy)-3-methoxy-2-mtrophenyl)-4-methyl-lH-imidazol-2- yl)-5-methoxypyπdme (0 15 g, 0 369 mmol) was dissolved in a solution containing ethanol (2 mL) and glacial acetic acid (2 mL) To this was added Fe powder (0 0 124 g, 2 2 mmol) The reaction was heated to 1000C for 1 h, the reaction was poured into IN NaOH and extracted with ethyl acetate The organic layer was separated and washed with water, bπne and dπed over MgS(It The solution was filtered and the solvent removed under reduced pressure 130 mg of an off white solid was recovered MS [(+)ESI] m/z = 377 1 [M-H]+
[0705]Step 7 8-(dιfluoromethoxy)-6-methoxy-l-(5-methoxypyndιn-3-yl)-3-methylιmιdazo[5, 1- c][1, 2, 4]benzotrιazιne
[0706]4-(difluoromethoxy)-2-methoxy-6-(2-(5-methoxypyridm-3-yl)-4-methyl-lH- imidazol-l-yl)anilme (0 12 g, 0 319 mmol) was dissolved in glacial acetic acid (4 mL) To this was added sodium mtπte (24 mg, 0 33 mmol) The reaction was stirred at RT for 1 h, then poured into IN NaOH and extracted with ethyl acetate The organic layer was separated, washed with water then bπne, dπed over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude recrystallized from ethyl acetate/ hexane
[0707]50 mg ofa yellow solid was recovered [(+)ESI] m/z = 388 1 [M+H]+ 1H NMR (400
20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
[0708]MHz, d6-DMSO) δ ppm 8 51 (s, IH), 8 47 (s IH), 7 76 (s, IH), 7 19 (t, J = 72 7, IH), 7 07 (m, IH), 6 52 (s, IH), 4 08 (s, 3H), 3 86 (s, 3H), 2 77 (s, 3H)
[0709]Example 171: l,8-Bis-(2,5-dichloro-phenyI)-3-methyl-imidazo[5,l- c] [1,2,4J benzotriazine
[0710]
[0711]Step 1 l-bromo-8-chloro-3-methylbenzo[e]ιmιdazo[5, l-c][l,2, 4]trιazme
[0712] This compound was prepared as descπbed in Example 132, step 6 by replacing 6- chloro-3-methyl-8-(tπfluoromethyl)benzo[e]imidazo[5,l-c][l,2,4]tπazme with 8-chloro- 3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazme (Example 42)
This compound was prepared as descπbed in Example 132, step 6 by replacing 6- chloro-3-methyl-8-(tπfluoromethyl)benzo[e]imidazo[5,l-c][l,2,4]tπazme with 8-chloro- 3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazme (Example 42)
[0713]Step 2 1 ,8-Bιs-(2,5-dιchloro-phenyl)-3-methyl-ιmιdazo[5,l-c] [1 2 ,4] benzotriazine
[0714]211 mg of l-bromo-8-chloro-3-methylbenzo[e]imidazo[5,l-c][l,2,4]tnazme was dissolved in 25 ml dioxane An second solution of 1 g K2CO3 in 3 2 ml water is added To this mixture 350 mg 2,5-dichlorophenyl boronic acid and 200 mg Pd(PPh3)4 are added The resulting mixture is heated up to reflux for 3 h At RT the solution is filtered off The solvent is distilled off from the clear solution The remaining residue is stirred with 50 ml water for 30 minutes The product suspension stays overnight The crude product is collected by filtration and purified by flash chromatography Yield 105 mg, MS [M+H]+ 473, m p 190-1930C
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0715]The following compounds of Table 8 were prepared analogous to the procedure for Example 171
[0718]Examples 179: l-Cyclohexyl-8-(2-cyclohexyl-4-methyl-imidazol-l-yl)-3-methyl- imidazo[5,l-c] [l,2,4]benzotriazine
[0719]
[0720]Step 1: 4-fluoro-2-(2-cyclohexyl-4-methyl-imidazol-l-yl)-nitrobenzene
[0721]
[0722]To a suspension of K2CO3 (6 g), 2-cyclohexyl-4-methyl imidazole (3 g) and 50 ml acetonitrile was added 2,4-difluoro-nitrobenzene (3.2 g). The reaction mixture was stirred and heated to reflux for 5 h. Then the reaction mixture was filtered off. The solvent was removed and the crude residue was purified by chromatography (DCM/methanol 96:4). Yield: 2.0 g
[0723]20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0724]Step 2 2,4-bιs-(2-cyclohexyl-4-methyl-ιmιdazol-l-yl)-mtrobenzene
[0725]
[0726]To a suspension of CSCO3 (6 g), 2-cyclohexyl-4-methyl imidazole (3 g) and 50 ml acetomtπle was added 4-fluoro-2-(2-cyclohexyl-4-methyl-imidazol-l-yl)-mtrobenzene (2 0 g) The reaction mixture was stirred and heated to reflux for 7 h Then the reaction mixture was filtered off The solvent was removed and the crude residue was purified by chromatography (DCM/methanol 96 4) Yield 1 25 g
[0727]Step 3 l-amιno-2,4-bιs-(2-cyclohexyl-4-methyl-ιmιdazol-l-yl)-benzene
[0728]
[0729]A mixture of 2,4-bis-(2-cyclohexyl-4-methyl-imidazol-l-yl)-mtrobenzene (1 25 g), 15 ml methanol, 3 ml water, 1 5 ml hydrazine hydrate and Raney-Ni catalyst (1 g) was stirred at room temperature for 2 hours During this time an additional portion of 0 5 ml hydrazine hydrate was added after 30 minutes and a second portion of 0 5 ml hydrazine hydrate after 1 hour The catalyst was filtered off 30 ml water and 100 ml ethyl acetate were added to the reaction mixture which was stirred again for 30 minutes The organic layer was separated and the solvent was removed The residual crude product is used without further purification Yield 1 0 g
[0730]Step 4 l-Cyclohexyl~8-(2-cyclohexyl-4-methyl-ιmιdazol-l-yl)-3-methyl-ιmιdazo[5,l-c]- [1, 2, 4] -benzotriazme
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT l-ammo-2,4-bis-(2-cyclohexyl-4-methyl-imidazol-l-yl)-benzene (1 0 g) was stirred with 15 ml IM H2SO4 at 00C A solution of sodium mtπte (0 8g) in 10 ml water was added to the solution over a peπod of 30 minutes The mixture was stirred for additional 2 hours at about 0 °C 10 ml water were added The mixture is neutralized with solid NaHCO3 stepwise to pH = 9 The crude product precipitated It was separated and washed with 20 ml water The product was purified by chromatography (DCM/methanol 95 5) Yield O 25 g , m p 224-2270C, MS[M+H]+ = 429
[0731]Examples 180: l-(2,5-Dichloro-phenyl)-3-methyl-8-piperidin-l-yl-imidazo[5,l- cj[l,2,4]benzotriazine
[0732]
[0733]This compound was prepared according to the procedure descπbed for Example 179 by replacing 2-cyclohexyl-4-methyl imidazole with 2-(2,5-dichlorophenyl)-4-methyl imidazole (from Example 5, step 1) in step 1 and by replacing 2-cyclohexyl-4-methyl imidazole with pipeπdme in step 2 m p 158-1600C, MS[M+H]+ = 412
[0734]Examples 181: l-(2,5-Dichloro-phenyl)-3-methyI-8-morpholin-4-yI-imidazo[5,l- c] [1,2,4] benzotriazine
[0735] This compound was prepared according to the procedure descnbed for Example
This compound was prepared according to the procedure descnbed for Example
[0736]179 by replacing 2-cyclohexyl-4-methyl imidazole with 2-(2,5-dichlorophenyl)-4-methyl imidazole (from Example 5, step 1) in step 1 and by replacing 2-cyclohexyl-4-methyl imidazole with morpholme in step 2 m p 239-2420C, MS[M+H]+ = 414
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
[0737]Examples 182: l-Isobutyl-8-(2-isobutyl-4-methyl-imidazol-l-yl)-3-methyl- imldazo[5,l-c] [l,2,4]benzotriazine
[0738]
[0739]This compound was prepared according to the procedure described for Example 179 by replacing 2-cyclohexyl-4-methyl imidazole with 2-(iso-butyl)-4-methyl imidazole in steps 1 and 2 m p 134-1360C, MS[MH-H]+ = 377
[0740]Examples 183: l-(2-Chloro-phenyl)-3-methyl-8-piperidin-l-yl-imidazo[5,l- c] [l,2,4]benzotriazine
[0741]
[0742]This compound was prepared according to the procedure descπbed for Example 179 by replacing 2-cyclohexyl-4-methyl imidazole with 2-(2-chlorophenyl)-4-methyl imidazole in step 1 and by replacing 2-cyclohexyl-4-methyl imidazole with pipendme in step 2 m p 207-2100C, MS[M+H]+ = 378
[0744]The following compounds of Table 9 were prepared analogous to the procedure for Example 183
[0746] 20743-0032WO1 (AM103539; 44418; HUBR- 1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR- 1344 PCT) PATENT
[0747]
[0748]Certain compounds of formula (I) are inhibitors of the enzyme PDE2 A substance is considered to effectively inhibit PDE2 if it has an IC50 of less than 10 μM, preferably less than 1 μM Certain compounds of formula (I) are inhibitors of the enzyme PDElO A substance is considered to effectively inhibit PDElO if it has an IC50 of less than 10 μM, preferably less than 1 μM
[0749]Example A: Inhibition of recombinant PDE2A (expressed in baculovirus/SF21- cells) PDE2A (NM002599) was cloned and the gene was inserted in the baculovirus and the enzyme-protem expressed in SF21 -cells The enzyme was isolated from these cells by harvesting the cells by an centnfugation at 200 g to collect the cells The cells were resuspended in 50 mM Tπs-HCl/5 mM MgC12 buffer (pH=7 4) (Sigma, Deisenhofen, Germany, Merck, Darmstadt, Germany) and lysed by a soni cation of the cells (three times for 15 seconds, Labsonic U, Fa Braun, Degersheim, Switzerland, level ,,high") The membrane fraction of PDE2A was obtained by a centnfugation at 48 000 g for 1 h, resuspended in buffer and stored at -70CC
[0750]PDE2A activity was determined in a one step procedure m microtiterplates The reaction mixture of 100 μl contained 50 mM Tπs-HCI/5 mM MgC12 buffer (pH=7 4)
20743-0032WO1 (AM103S39, 44418; HUBR-1344 PCT) PATENT
[0751](Sigma, Deisenhofen, Germany, Merck, Darmstadt, Germany), 0 5 μM [3H]-cAMP (Amersham, Buckinghamshire, UK), 100OnM cGMP and the enzyme Non-specific enzyme activity was tested in the absence of cGMP The reaction was initiated by addition of the substrate solution and was earned out at 37 "C for 30 minutes Enzymatic activity then was stopped by addition of 25 μl SPA-beads (Amersham-Pharmacia) One hour later the mixture was measured in a liquid scintillation counter for microtiterplates (Microbeta Tπlux) For pipetting of the incubation mixture the robot Biomek (Fa Beckman) was routinely used
[0752]The determined ICm for this assay was Kn, = 4200 nmol/1 for the membrane fraction and Kn, = 5300 nM for the cytosolic fraction The optimal amount of enzyme m the assay has been determined and optimised for each enzyme preparation separately before using the enzyme in compound testing For determination of IC50 values the Hill- plot, 2-parameter-model, was used
[0753]Example B: Inhibition of recombinant PDElOA (baculovirus/SF21 system)
[0754]The DNA of PDElOAl (AB 020593, 2340 bp) was synthesized and cloned into the vector pCR4 TOPO (Entelechon GmbH, Regensburg, Germany) The gene was than inserted into a baculovirus vector, hgated with the baculovirus DNA The enzyme-protein was expressed m SF21 -cells The enzyme was isolated from these cells by harvesting the cells by an centπfugation at 500 g to collect the cells
[0755]The cells were resuspended m 50 mM Tns-HCl/1 mM EDTA/250mM Sucrose buffer, pH=7 4 (Sigma, Deisenhofen, Germany, Merck, Darmstadt, Germany) and lysed by somfication of the cells (three times for 15 seconds, Labsomc U, Fa Braun, Degersheim, Switzerland, level ,,high") The cytosolic PDElOA was obtained by a centπfugation at 48,000 g for 1 h in the supernatant and stored at -700C
[0756]PDE activity was determined in a one step procedure in microtiter plates The reaction mixture of 100 μl contained 50 mM Tπs-HCl/5 mM MgC12 buffer (pH=74, Sigma, Deisenhofen, Germany, Merck, Darmstadt, Germany) 0 1 μM [3H]-cAMP (Amersham, Buckinghamshire, UK) and the enzyme Non-specific enzyme activity was determined without the enzyme The reaction was initiated by addition of the substrate solution and was earned out at 37 °C for 30 minutes Enzymatic activity then was
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT stopped by addition of 25 μl Ysi-SPA-beads (Amersham-Pharmacia) One hour later the mixture was measured in a liquid scintillation counter for microtiter plates (Microbeta Tπlux) The Biomek 2000 (Beckman) was used routinely for pipetting of the incubation mixture The optimal amount of enzyme in the assay has been determined and optimized for each enzyme preparation separately before using the enzyme in compound testing For determination of IC50 values the Hill-plot, 2-parameter-model, was used
[0757]Table Of IC50 data for PDE2A and PDElOA assays*
[0758]
 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0759]
 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0760]

[0761]The compounds of formula (I) show significant antidepressant, anxiolytic and cognition enhancing effects in vivo
[0762]Example C. Novel object recognition.
[0763]The novel object recognition is an animal model of learning and memory (Rutten et al , 2006a+b)
[0764]The novel object recognition is performed m glass aquaria (40 x 60 x 40 cm) that have 3 black walls and one transparent wall The floor consists of black, antishp PVC Objects of different material (iron, plastic, coated hardwood) and forms and similar size are used for the experiment The objects are positioned 10 cm from the wall and 35-40 cm from each other Female Wistar-rats are used for this experiment
[0765]One day before the experiment rats have 15 nun to habituate to the arena and two objects On the first day of the expeπment rats are placed into the arena and have five mm to explore two equal objects To disturb the learning process, MK-801 at 0 025 mg/kg is
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT administered mtrapentoneally on the first day of the experiment 30 mm before the test starts
[0766]On the second day of the expeπment (24h later) rats are again placed into the arena and have 5 mm to explore one of the old objects and a novel object The position of the novel object is changed from rat to rat to avoid a place preference
[0767]The following parameters are recorded
[0768]1 the time the rats spent with each object on the first day
[0769]2 the time the rats spent with each object on the second day
[0770]3 percent of time rats spent with the novel object on the second day Exploratory contact is regarded as the nose of the rat being withm a 2-cm-radius of an object
[0771]Vehicle or compounds of formula (I) are given orally as a suspension on the first day of expeπment 30 mm prior to the test session
[0772]Example D. Forced swim test.
[0773]The forced swim test is an established animal model of depression (Yacoubi et al , 2001) Mice which are forced to swim in a restricted area from which they cannot escape will rapidly cease attempts to escape and adopt a characteristic immobile posture which can be readily identified and timed Immobility is taken as depression-related behaviour in the animal (Porsolt, 1979)
[0774]For the test a glass cylinder (height 20 cm, internal diameter 15 cm) containing 11 cm water maintained at 230C is used On the day of expeπment the mice are forced to swim in the water for 6 min and the immobility time is recorded dunng the last 4 mm of the 6-mm-penod Afterwards animals are removed from the water, dried with a paper towel and put under infrared light
[0775]Example E. Light and dark box.
[0776]The light and dark box is an established animal model of anxiety (Crawley, 1985) The light and dark box consists of two chambers (each 30x30 cm) that are connected by an opening There is an aversive chamber with white walls that is bnghtly lit (600 lux) and a dark chamber with black walls that is only lit by an infrared lamp (150 lux)
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0777]Untreated mice predominately stay in the dark chamber whereas mice treated with an anxiolytic compound go more often into the light chamber resulting in an increased number of transitions between the boxes and increased time in the light box In addition the distance traveled in the dark chamber is regarded as an activity-related parameter For the experiment, mice are placed m the light box after the pre-treatment time
[0778]Recording time starts when the mouse enters the dark box for the first time Then the animal has 5 mm to explore the two chambers
[0779]The behaviour of the mice is recorded by video and analyzed by VideoMot 2 (TSE systems, Germany) The following parameters are recorded 1 number of transitions [n] as anxiety-related parameter
[0780]2 distance traveled m the dark chamber [cm] as activity-related-parameter
[0781]Example F. Statistics.
[0782]Results are analyzed by t-test (two groups) or one way analysis of variance (ANOVA) when several groups are compared Tukey test is used for individual comparison P < 0 05 is regarded as significant
[0784]Antonim I, Franchetti P, Gπfantim M, Martelh S (1976) A new example of intramolecular 1,5-hydrogen transfer during diazotization reactions J Org Chem 41 158-159
[0785]Baddeley A (2003) Working memory looking back and looking forward Nat Rev Neurosci 4 (10) 829-839
[0786]Beck AT (2008) The evolution of the cognitive model of depression and its neurobiological correlates Am J Psychiatry 165 969-977
[0787]Blokland A, Schreiber R, Prickaerts J (2006) Improving memory a role for phosphodiesterases Curr Pharrn Des 12 (20) 2511-2523
[0788]Boess FG, Hendnx M, van der Staay FJ, Erb C, Schreiber R, van Staveren W, de Vente J, Prickaerts J, Blokland A, Koemg G (2004) Inhibition of phosphodiesterase 2
20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT increases neuronal cGMP, synaptic plasticity and memory performance Neuropharmacology 47 (7) 1081-1092
[0789]Bolger GB, Rodgers L, Riggs M (1994) Differential CNS expression of alternative mRNA isoforms of the mammalian genes encoding cAMP-specifϊc phosphodiesterases Gene 149 237-44
[0790]Conti M, Jm SL (1999) The molecular biology of cyclic nucleotide phosphodiesterases Prog Nucleic Acid Res MoI Biol 63 1-38
[0791]Crawley JN (1985) Exploratory Behavior Models of Anxiety in Mice Neurosci Biobehav Rev 9 37-44 Duman RS (1998) Novel therapeutic approaches beyond the serotonin receptor
[0792]Biol Psychiatry 44 (5) 324-335
[0793]Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg- Thompson S (2000) Executive dysfunction predicts nonresponse to fluoxetine in major depression J Affect Disord 60 13-23 ElYacoubi M, Ledent C, Parmentier M, Bertorelli R, Ongim E, Costentm J, and
[0794]Vaugeois JM (2001) Adenosine A2A receptor antagonists are potential antidepressants evidence based on pharmacology and A2A receptor knockout mice Br J Pharmacol 134 68-77
[0795]Essayan DM (2001) Cyclic nucleotide phosphodiesterases J Allergy CIm Immunol 108 671-680
[0796]Gualtieπ CT, Johnson LG, Benedict KB (2006) Neurocognition m depression patients on and off medication versus healthy comparison subjects J Neuropsychiatry CIm Neurosci 18 217-225
[0797]Gorlyn M, Keilp JG, Grunebaum MF, Taylor BP, Oquendo MA, Bruder GE, Stewart JW, Zalsman G, Mann JJ (2008) Neuropsychological characteristics as predictor of SSRI treatment response in depressed subjects J Neural Transm 115 1213-1219
[0798]Kumar S, Kulkarni SK (1996) Influence of antidepressant drugs on learning and memory paradigms m mice Indian J Exp Biol 34 431-435
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0799]Lakics et al Society of Neuroscience 35th Annual Meeting, 2005, Nov 12-16th, Wahmgton DC
[0800]Mandelli L, Serretti A, Colombo C, Floπta M, Santoro A, Rossini D, Zanardi R, Smeraldi E (2006) Improvement of cognitive functioning in mood disorder patients with depressive symptomatic recovery duπno treatment an exploratory analysis Psychiatry Clin Neurosci 60 598-604
[0801]Masood A, Nadeem A, Mustafa SJ, O'Donnell JM (2008) Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 m mice JPET 326 369-379
[0802]Menniti FS, Faraci WS, Schmidt CJ (2006) Phosphodiesterases in the CNS targets for drag development Nat Rev Drug Discov 5 (8) 660-670
[0803]Modell JG, Mountz JM, Beresford TP (1990) Basal ganglia/limbic stπatal and thalamocortical involvement in craving and loss of control m alcoholism J Neuropsychiatry CIm Neurosci 2 (2) 123-144
[0804]Nibuya M, Nestler EJ, Duman RS (1996) Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus J Neurosci 16 (7) 2365-2372
[0805]Paelecke-Habermarm Y, Pohl J, Leplow B (2005) Attention and executive functions in remitted major depression patients J Affect Disord 89 125-135
[0806]Porsolt RD (1979) Animal model of depression Biomedicme 30 139-40 Pπckaerts J, de Vente J, Homg W, Stembusch HW, Blokland A (2002) cGMP, but not cAMP, in rat hippocampus is involved in early stages of object memory consolidation Eur J Pharmacol 436 (1-2) 83-87
[0807]Ramanathan M, Kumar SN, Suresh B (2003) Evaluation of cognitive function of fluoxetine, sertraline and tianeptine in isolation and chronic unpredictable mild stress- induced depressive Wistar rats Indian J Exp Biol 41 1269-1272
[0808]Rutten K, Pπckaerts J, Blokland A (2006a) Rolipram reverses scopolamine- mduced and time-dependent memory deficits in object recognition by different mechanisms of action Neurobiol Learn Mem 85 132-138
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0809]Rutten K, Pnckaerts J, Hendπx M, van der Staay FJ, Sik A, Blokland A (2006b) Time-dependent involvement of cAMP and cGMP in consolidation of object memory studies using selective phosphodiesterase type 2, 4 and 5 inhibitors Eur J Pharmacol 558 107-112 Sadchikova E V, et all (2000) Synthesis of new heteroaromatic systems naphtha[2,l-e]imidazo[5,l-c]l,2,4-tπazmes and benz[e]imidazo[5,l-c]-l,2,4-tπazines Chemistry of Heterocyclic Compounds 36 465-471
[0810]Sadchikova E V and Mokrushin V S (2005) Reactivity of diazoles and azolediazomum salts in C-azo coupling reactions Russian Chemical Bulletin, International Edition 54 354-365
[0811]Shelton RC, Mamer DH, Peterson CS3 Ellis TC, Sulser F (1999) Cyclic AMP- dependent protein kinase in subtypes of major depression and normal volunteers Int J Neuropsychopharmacol 2 (3) 187-192
[0812]Simonov A M, Sitkma L M, Pozharskn A F (1967) Unusual reaction of azo- couplimg m the imidazole seπes Chemistry & Industry 34 1454
[0813]Simonov A M et all (1969) Unusual reaction of azo-couphmg m an imidazole seπes Formation of a new heterocyclic system of imidazo-l,2,4-benzotnazme Khimia Geterotsiklicheskikh Soedimemi 5 916-922
[0814]Soderlmg,S H and BeavoJ A (2000) Regulation of cAMP and cGMP signaling new phosphodiesterases and new functions Curr Opin Cell Biol 12 174-179
[0815]Valluzi JA, Chan K (2007) Effect of fluoxetine on hippocampal-dependent and hippocampal-mdependent learning tasks Behav Pharmacol 18 507-513
[0816]Van Staveren WC, Stembusch HW, Markerink-Van Ittersum M, Repaske DR, Goy MF, Kotera J, Omoπ K, Beavo JA, De Vente J (2003) mRNA expression patterns of the cGMP-hydrolyzing phosphodiesterases types 2, 5, and 9 during development of the rat brain J Comp Neurol 467 566-80
[0817]This application claims the benefit of priority of U S Provisional Application No
[0818]61/198,695, which is incorporated herein by reference in its entirety
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
[0819]A number of embodiments of the invention have been descnbed Nevertheless, it will be understood that vaπous modifications may be made without departing from the spint and scope of the invention



 I or a pharmaceutically acceptable salt thereof, wherein a, b, c, and d indicate four possible positions on the ring for each R3, when present, p is 0 or an integer from 1 to 4,
I or a pharmaceutically acceptable salt thereof, wherein a, b, c, and d indicate four possible positions on the ring for each R3, when present, p is 0 or an integer from 1 to 4, and -P(O)(OR4)2, wherein q is 1 or 2,
and -P(O)(OR4)2, wherein q is 1 or 2, and -P(O)(OR4)2
and -P(O)(OR4)2
 Ic or a pharmaceutically acceptable salt thereof
Ic or a pharmaceutically acceptable salt thereof I or a pharmaceutically acceptable salt thereof, wherein a, b, c, and d indicate four possible positions on the ring for each R3, when present, p is 0 or an integer from 1 to 4,
I or a pharmaceutically acceptable salt thereof, wherein a, b, c, and d indicate four possible positions on the ring for each R3, when present, p is 0 or an integer from 1 to 4,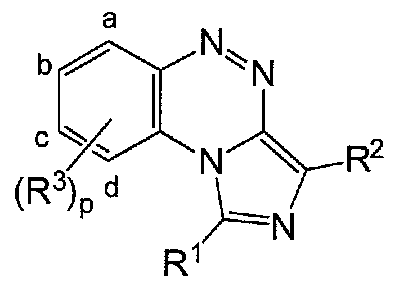 I or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable earner, wherein a, b, c, and d indicate four possible positions on the πng for each R3, when present, p is 0 or an integer from 1 to 4,
I or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable earner, wherein a, b, c, and d indicate four possible positions on the πng for each R3, when present, p is 0 or an integer from 1 to 4,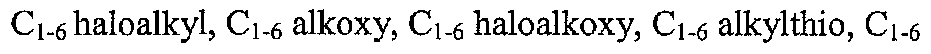 Cue alkylthio, C^ alkylsulfinyl, Ci.6 alkylsulfonyl, amino, Ci_6 alkylamino, di-Ci-β-alkylamino, Ci_6 alkylcarbonyl, Ci_s alkoxycarbonyl, carboxy, carbamyl, Ci_6 alkylcarbamyl, di-Ci-6 alkylcarbamyl, Ci_6 alkylcarbamyloxy, and di-Ci-6-alkylcarbamyloxy.
Cue alkylthio, C^ alkylsulfinyl, Ci.6 alkylsulfonyl, amino, Ci_6 alkylamino, di-Ci-β-alkylamino, Ci_6 alkylcarbonyl, Ci_s alkoxycarbonyl, carboxy, carbamyl, Ci_6 alkylcarbamyl, di-Ci-6 alkylcarbamyl, Ci_6 alkylcarbamyloxy, and di-Ci-6-alkylcarbamyloxy.




 and -P(O)(OR4)2
and -P(O)(OR4)2 or a pharmaceutically acceptable salt thereof
or a pharmaceutically acceptable salt thereof or a pharmaceutically acceptable salt thereof
or a pharmaceutically acceptable salt thereof
 then it is understood that substituent R can occur p number of times on the πng, and R can be a different moiety at each occurrence It is understood that each R group may replace any hydrogen atom attached to a πng atom, including one or both of the (CH2)n hydrogen atoms Further, in the above example, should the variable Q be defined to include hydrogens, such as when Q is said to be CH2, NH, etc , any floating substituent such as R in the above example, can replace a hydrogen of the Q vanable as well as a hydrogen m any other non- variable component of the πng
then it is understood that substituent R can occur p number of times on the πng, and R can be a different moiety at each occurrence It is understood that each R group may replace any hydrogen atom attached to a πng atom, including one or both of the (CH2)n hydrogen atoms Further, in the above example, should the variable Q be defined to include hydrogens, such as when Q is said to be CH2, NH, etc , any floating substituent such as R in the above example, can replace a hydrogen of the Q vanable as well as a hydrogen m any other non- variable component of the πng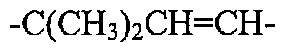 and -CH-(C2H5)-CH=CH-
and -CH-(C2H5)-CH=CH-



 Step 1 4-fluoro-2-(4-methyl-2-propyl-ιmιdazol-l-yl)-nιtrobenzene
Step 1 4-fluoro-2-(4-methyl-2-propyl-ιmιdazol-l-yl)-nιtrobenzene

 l-amino-4-fluoro-2-(4-methyl-2-propyl-imidazol-l-yl)-benzene (2 g) was dissolved in 25 ml of 1 M H2SO4 This solution was stirred and cooled with ice A solution of sodium nitrite (1 g) m 10 ml water was added to the mixture over a peπod of time of 30 minutes It was stirred for additional 2 h at about 00C A crude yellow product precipitated It was filtered off and re-crystallized from iso-propanol Yield 3 6 g, m p 124 5-126 50C, MS [M+H]+ 245
l-amino-4-fluoro-2-(4-methyl-2-propyl-imidazol-l-yl)-benzene (2 g) was dissolved in 25 ml of 1 M H2SO4 This solution was stirred and cooled with ice A solution of sodium nitrite (1 g) m 10 ml water was added to the mixture over a peπod of time of 30 minutes It was stirred for additional 2 h at about 00C A crude yellow product precipitated It was filtered off and re-crystallized from iso-propanol Yield 3 6 g, m p 124 5-126 50C, MS [M+H]+ 245 20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
 6 5 g cyclohexyl aldehyde was stirred with 44 ml ethanol and 23 ml cone
6 5 g cyclohexyl aldehyde was stirred with 44 ml ethanol and 23 ml cone
 10 0 g 2,5-dichlorobenzaldehyde was stirred with 44 ml ethanol and 23 ml cone
10 0 g 2,5-dichlorobenzaldehyde was stirred with 44 ml ethanol and 23 ml cone

 20743-0032WO1 (AM103539, 44418, HUBR- 1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR- 1344 PCT) PATENT 20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT 20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT


 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT


 20743-0032WO1 (AM103S39; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103S39; 44418; HUBR-1344 PCT) PATENT



 To a mixture of 2-bromo-l-(3,5-dimethoxy-2-mtrophenyl)-4-methyl-liy- lmidazole (1 1 g, 3 2 mmol), 4-methyl-3-pyπdylboromc acid (commercially available
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT from Combi-Blocks Inc , 876 mg, 6 4 mmol), K2CO3 (1 32 g, 9 6 mmol) and Pd(PPh3)4 (186 mg, 0 161 mmol) in a 20 mL vial was added 60 mL of 1 ,4-dioxane and 20 mL of water under nitrogen The mixture was stirred at 900C for 4 h and cooled to RT Standard work up followed by column purification provided 788 mg (69% yield) of the desired product as an off-white powder EMS 355 1 [M+H]+
To a mixture of 2-bromo-l-(3,5-dimethoxy-2-mtrophenyl)-4-methyl-liy- lmidazole (1 1 g, 3 2 mmol), 4-methyl-3-pyπdylboromc acid (commercially available
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT from Combi-Blocks Inc , 876 mg, 6 4 mmol), K2CO3 (1 32 g, 9 6 mmol) and Pd(PPh3)4 (186 mg, 0 161 mmol) in a 20 mL vial was added 60 mL of 1 ,4-dioxane and 20 mL of water under nitrogen The mixture was stirred at 900C for 4 h and cooled to RT Standard work up followed by column purification provided 788 mg (69% yield) of the desired product as an off-white powder EMS 355 1 [M+H]+

 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT

 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT


 To a mixture of l-bromo-6-fluoro-8-methoxy-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tπazine (1 mmol), boronic acid (commercially available from Sigma-Aldnch Corporation, Boron Molecular Inc , SynQuest Laboratoπes, and Combi-Blocks Inc , 2 mmol), K2CO3 (3 mmol) and Pd(PPh3)4 (O 05 mmol) was added 20 mL of 1,4-dioxane and 7 mL of water under nitrogen The mixture was stirred at 90 °C for 4 h and cooled to RT Standard work up followed by column purification provided (70-80% yield) of the desired product
To a mixture of l-bromo-6-fluoro-8-methoxy-3-methylbenzo[e]imidazo[5,l- c][l,2,4]tπazine (1 mmol), boronic acid (commercially available from Sigma-Aldnch Corporation, Boron Molecular Inc , SynQuest Laboratoπes, and Combi-Blocks Inc , 2 mmol), K2CO3 (3 mmol) and Pd(PPh3)4 (O 05 mmol) was added 20 mL of 1,4-dioxane and 7 mL of water under nitrogen The mixture was stirred at 90 °C for 4 h and cooled to RT Standard work up followed by column purification provided (70-80% yield) of the desired product
 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT 18
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT 18
 20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418, HUBR-1344 PCT) PATENT

 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT 20743-0032WO1 (AMI 03539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AMI 03539; 44418; HUBR-1344 PCT) PATENT
 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT


 Sodium perborate tetrahydrate (7 3 g, 46 8 mmol) was suspended in glacial acetic acid (30 mL) and heated to 500C To this was added dropwise a solution of 2-chloro-6- fluoro-4-(tnfluoromethyl)amline (2 g, 9 37 mmol) dissolved m glacial acetic acid (20 mL) The reaction was stirred for 16 h at 500C The reaction was then poured into water and extracted with ether The organic layer was separated and washed with water, then dilute aqueous bicarbonate solution and bπne The organic layer was then dried over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in hexane 1 17 g of a brown oil was recovered MS [(+)ESI] m/z = 242 8 [M-H]+
Sodium perborate tetrahydrate (7 3 g, 46 8 mmol) was suspended in glacial acetic acid (30 mL) and heated to 500C To this was added dropwise a solution of 2-chloro-6- fluoro-4-(tnfluoromethyl)amline (2 g, 9 37 mmol) dissolved m glacial acetic acid (20 mL) The reaction was stirred for 16 h at 500C The reaction was then poured into water and extracted with ether The organic layer was separated and washed with water, then dilute aqueous bicarbonate solution and bπne The organic layer was then dried over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in hexane 1 17 g of a brown oil was recovered MS [(+)ESI] m/z = 242 8 [M-H]+ l-Chloro-3-fluoro-2-mtro-5-(tnfluoromethyl)benzene (1 1 g, 4 5 mmol) and 4- methyl-lH-irmdazole (from Sigma-Aldπch Co , 0 371 g, 4 5 mmol) were dissolved m DMF (IO mL) To this was added potassium carbonate (1 2 g, 9 0 mmol) The reaction was let stir at RT for 16 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water then bπne and dπed
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT over MgSθ4 The solution was then filtered and the solvent removed under reduced pressure The crude was purified by flash chromatography on silica gel m 10 2 hexane/ ethyl acetate 0 74 g of a tan solid was collected MS [(+)ESI] m/z = 306 0 [M-H]+
l-Chloro-3-fluoro-2-mtro-5-(tnfluoromethyl)benzene (1 1 g, 4 5 mmol) and 4- methyl-lH-irmdazole (from Sigma-Aldπch Co , 0 371 g, 4 5 mmol) were dissolved m DMF (IO mL) To this was added potassium carbonate (1 2 g, 9 0 mmol) The reaction was let stir at RT for 16 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water then bπne and dπed
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT over MgSθ4 The solution was then filtered and the solvent removed under reduced pressure The crude was purified by flash chromatography on silica gel m 10 2 hexane/ ethyl acetate 0 74 g of a tan solid was collected MS [(+)ESI] m/z = 306 0 [M-H]+ l-(3-Chloro-2-nitro-5-(tπfluoromethyl)phenyl)-4-methyl-lH-imidazole (0 74 g, 2 4 mmol) was dissolved in a solution of glacial acetic acid and ethanol (10 mL each) To this was then added iron powder (081 g, 144 mmol) The reaction was heated to 1000C for 1 h The reaction was poured into aqueous sodium hydroxide IN and extracted with ethyl acetate The organic layer was separated then washed with water, bπne and dπed over MgSO4 The resulting solution was filtered and the solvent removed under reduced pressure An off white solid (0 68 g) was recovered MS [(+)ESI] m/z = 274 1 [M-H]+
l-(3-Chloro-2-nitro-5-(tπfluoromethyl)phenyl)-4-methyl-lH-imidazole (0 74 g, 2 4 mmol) was dissolved in a solution of glacial acetic acid and ethanol (10 mL each) To this was then added iron powder (081 g, 144 mmol) The reaction was heated to 1000C for 1 h The reaction was poured into aqueous sodium hydroxide IN and extracted with ethyl acetate The organic layer was separated then washed with water, bπne and dπed over MgSO4 The resulting solution was filtered and the solvent removed under reduced pressure An off white solid (0 68 g) was recovered MS [(+)ESI] m/z = 274 1 [M-H]+
 δ-Chloro-S-methyl-δ^tnfluoromemytybeiizofelirnidazofSJ-cJfl^^triazme (045 g, 1 57 mmol) was dissolved in acetomtnle (20 mL) To this was added N- bromosuccmamide (1 1 g, 6 3 mmol)) The reaction was covered from light and stirred at RT for 48 h The reaction was poured into water and extracted with chloroform The organic layer was separated then bπne and dried over MgSO4 and filtered The solvent was removed under reduced pressure and the crude put on silica gel in 10 1 hexane/ethyl acetate 043 g of a yellow solid was recovered MS [(+)ESI] m/z = 364 9 [M-H]+
δ-Chloro-S-methyl-δ^tnfluoromemytybeiizofelirnidazofSJ-cJfl^^triazme (045 g, 1 57 mmol) was dissolved in acetomtnle (20 mL) To this was added N- bromosuccmamide (1 1 g, 6 3 mmol)) The reaction was covered from light and stirred at RT for 48 h The reaction was poured into water and extracted with chloroform The organic layer was separated then bπne and dried over MgSO4 and filtered The solvent was removed under reduced pressure and the crude put on silica gel in 10 1 hexane/ethyl acetate 043 g of a yellow solid was recovered MS [(+)ESI] m/z = 364 9 [M-H]+


 l-(3-fluoro-2-mtro-5-(tnfluoromethyl)phenyl)-4-methyl-lH-imidazole (02 g, 0 692 mmol) was dissolved in MeOH (3 mL) To this was added NaOMe/MeOH (25%) (2eq, 0 36 mL) The reaction darkened slightly and was stirred at RT for 2hrs The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water, bπne and dπed over MgSO4, then filtered The solvent was removed under reduced pressure 0 18 g ofa tan solid (90% yield) was recovered MS [(+)ESI] m/z = 302 1 [M-H]+
l-(3-fluoro-2-mtro-5-(tnfluoromethyl)phenyl)-4-methyl-lH-imidazole (02 g, 0 692 mmol) was dissolved in MeOH (3 mL) To this was added NaOMe/MeOH (25%) (2eq, 0 36 mL) The reaction darkened slightly and was stirred at RT for 2hrs The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water, bπne and dπed over MgSO4, then filtered The solvent was removed under reduced pressure 0 18 g ofa tan solid (90% yield) was recovered MS [(+)ESI] m/z = 302 1 [M-H]+ l-(3-methoxy-2-mtro-5-(tπfluoromethyl)phenyl)-4-methyl-lH-imidazole (0 18 g, 0 598 mmol) was dissolved in a solution containing ethanol and glacial acetic acid (4 mL each) To this was added iron powder (0 2 g, 6 eq) The reaction was heated to 1000C for 1 h then poured into aqueous NaOH and extracted with ethyl acetate The organic layer was separated and washed with water, bπne and dπed over MgSO4, then filtered and removed of solvent under reduced pressure A tan solid 0 15 g (94% yield) was recovered MS [(+)ESI] m/z = 272 1 [M-H]+
l-(3-methoxy-2-mtro-5-(tπfluoromethyl)phenyl)-4-methyl-lH-imidazole (0 18 g, 0 598 mmol) was dissolved in a solution containing ethanol and glacial acetic acid (4 mL each) To this was added iron powder (0 2 g, 6 eq) The reaction was heated to 1000C for 1 h then poured into aqueous NaOH and extracted with ethyl acetate The organic layer was separated and washed with water, bπne and dπed over MgSO4, then filtered and removed of solvent under reduced pressure A tan solid 0 15 g (94% yield) was recovered MS [(+)ESI] m/z = 272 1 [M-H]+


 l,3-difluoro-2-mtro-5-(tπfluoromethyl)benzene (prepared as described above procedure, Step 1, 1 98 g, 8 72 mmol) and 2-bromo-4-methyl-lH-imidazole (1 404 g, 8 72 mmol) were dissolved in DMF (20 mL) To this was added K2CO3 (2 eq, 2 4 g) The reaction was stirred at RT for 1 5 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water then bπne, dried over MgSC>4 and filtered The solvent was removed under reduced pressure and the crude was taken in CHCI3 The resulting precipitate was filtered and collected The mother liquor was purified by flash chromatography on silica gel in 10 1 to 10 2 hexane/ethyl acetate 1 76 g (55% yield) of a pale yellow solid was recovered MS K+)ESI] m/z = 260 0 [M-H]+
l,3-difluoro-2-mtro-5-(tπfluoromethyl)benzene (prepared as described above procedure, Step 1, 1 98 g, 8 72 mmol) and 2-bromo-4-methyl-lH-imidazole (1 404 g, 8 72 mmol) were dissolved in DMF (20 mL) To this was added K2CO3 (2 eq, 2 4 g) The reaction was stirred at RT for 1 5 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and washed with water then bπne, dried over MgSC>4 and filtered The solvent was removed under reduced pressure and the crude was taken in CHCI3 The resulting precipitate was filtered and collected The mother liquor was purified by flash chromatography on silica gel in 10 1 to 10 2 hexane/ethyl acetate 1 76 g (55% yield) of a pale yellow solid was recovered MS K+)ESI] m/z = 260 0 [M-H]+ 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT


 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT

 20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103S39, 44418, HUBR-1344 PCT) PATENT

 20743-0032WO1 (AM103S39, 44418, HϋBR-1344 PCT) PATENT
20743-0032WO1 (AM103S39, 44418, HϋBR-1344 PCT) PATENT

 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT

 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT

 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT




 A mixture of 4-(l-(5-(benzyloxy)-3-methoxy-2-mtrophenyl)-4-methyl-lH- imidazol-2-yl)-3-methylpyπdine (412 mg, 0 957 mmol) and tin(II)chloπde dihydrate (1080 mg, 479 mmol) in a 250 mL flask was vacuumed and refilled with nitrogen, followed by the addition of ethanol (10 mL) and hydrochloric acid (37%, 0 286 mL)
20743-0032WO1 (AM103539; 44418; HUBR- 1344 PCT) PATENT
A mixture of 4-(l-(5-(benzyloxy)-3-methoxy-2-mtrophenyl)-4-methyl-lH- imidazol-2-yl)-3-methylpyπdine (412 mg, 0 957 mmol) and tin(II)chloπde dihydrate (1080 mg, 479 mmol) in a 250 mL flask was vacuumed and refilled with nitrogen, followed by the addition of ethanol (10 mL) and hydrochloric acid (37%, 0 286 mL)
20743-0032WO1 (AM103539; 44418; HUBR- 1344 PCT) PATENT










 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT



 20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418; HUBR-1344 PCT) PATENT



 l,5-difluoro-3-methoxy-2-mtrobenzene (7 g, 37 0 mmol) and 4-methyl-lH- lmidazole (3 04 g, 37 0 mmol) were dissloved in DMF (100 mL). To this was added potassium carbonate (10 g, 74 mmol)) The reaction was stirred at RT overnight then poured into water and extracted with ethyl acetate The organic layer was separated, washed with water, bπne, then dπed over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
l,5-difluoro-3-methoxy-2-mtrobenzene (7 g, 37 0 mmol) and 4-methyl-lH- lmidazole (3 04 g, 37 0 mmol) were dissloved in DMF (100 mL). To this was added potassium carbonate (10 g, 74 mmol)) The reaction was stirred at RT overnight then poured into water and extracted with ethyl acetate The organic layer was separated, washed with water, bπne, then dπed over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT l-(5-fluoro-3-methoxy-2-mtrophenyl)-4-methyl-lH-imidazole (4 9 g, 19 51 mmol) was dissolved m THF (40 mL) and cooled to 0 °C To this was added 1- bromopyrrolidine-2,5-dione (3 65 g, 2048 mmol) The reaction was let warm to RT after 1 h and stirred for 4 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and combined then bπne, dried over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in 20% to 40% ethyl acetate/hexane 1 6 g of a waxy yellow solid was collected MS [(+)ESI] m/z = 330 0 [M-H]+
l-(5-fluoro-3-methoxy-2-mtrophenyl)-4-methyl-lH-imidazole (4 9 g, 19 51 mmol) was dissolved m THF (40 mL) and cooled to 0 °C To this was added 1- bromopyrrolidine-2,5-dione (3 65 g, 2048 mmol) The reaction was let warm to RT after 1 h and stirred for 4 h The reaction was poured into water and extracted with ethyl acetate The organic layer was separated and combined then bπne, dried over MgSθ4 and filtered The solvent was removed under reduced pressure and the crude purified by flash chromatography on silica gel in 20% to 40% ethyl acetate/hexane 1 6 g of a waxy yellow solid was collected MS [(+)ESI] m/z = 330 0 [M-H]+




 This compound was prepared as descπbed in Example 132, step 6 by replacing 6- chloro-3-methyl-8-(tπfluoromethyl)benzo[e]imidazo[5,l-c][l,2,4]tπazme with 8-chloro- 3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazme (Example 42)
This compound was prepared as descπbed in Example 132, step 6 by replacing 6- chloro-3-methyl-8-(tπfluoromethyl)benzo[e]imidazo[5,l-c][l,2,4]tπazme with 8-chloro- 3-methylbenzo[e]imidazo[5,l-c][l,2,4]tπazme (Example 42)
 20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR-1344 PCT) PATENT






 This compound was prepared according to the procedure descnbed for Example
This compound was prepared according to the procedure descnbed for Example

 20743-0032WO1 (AM103539; 44418; HUBR- 1344 PCT) PATENT
20743-0032WO1 (AM103539; 44418; HUBR- 1344 PCT) PATENT

 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
 20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT
20743-0032WO1 (AM103539, 44418, HUBR-1344 PCT) PATENT


