CROSS REFERENCE
[0001]This Application claims the benefit of U.S. Provisional Application No. 62/469,460, filed Mar. 9, 2017; U.S. Provisional Application No. 62/473,721, filed Mar. 20, 2017; and U.S. Provisional Application No. 62/477,741, filed Mar. 28, 2017, which are incorporated herein by reference in their entirety.
SEQUENCE LISTING
[0002]The instant application contains a Sequence Listing which has been submitted electronically in ASCII format and is hereby incorporated by reference in its entirety. Said ASCII copy, created on Mar. 27, 2018, is named 35224-820_201_SL.txt and is 1,594,927 bytes in size.
BACKGROUND OF THE INVENTION
[0003]Myeloid cell leukemia 1 (MCL-1) is a protein that inhibits cell death by binding and inhibiting pro-death factors, such as BCL-2 interacting mediator (BIM). BFL-1, an anti-apoptotic BCL-2 family member, blocks p53-mediated apoptosis and has oncogenic transforming activity.
INCORPORATION BY REFERENCE
[0004]All publications, patents, and patent applications mentioned in this specification are herein incorporated by reference in their entirety for all purposes, to the same extent as if each individual publication, patent, or patent application was specifically and individually indicated to be incorporated by reference.
SUMMARY OF THE INVENTION
[0005]In some embodiments, the invention provides a peptidomimetic macrocycle of Formula (Ic):
[0000]
[0006]each A, C, D, E, and F is independently a natural or non-natural amino acid;
[0007]each B is independently a natural or non-natural amino acid, amino acid analogue,
[0000]
[0000][—NH-L3-CO—], [—NH-L3-SO2—], or [—NH-L3-];
[0008]WH is an amino acid with an electron accepting group susceptible to attack by a nucleophile;
[0009]each L is independently a macrocycle-forming linker;
[0010]each L′ is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5, or a bond, or together with R1and the atom to which both R1and L″ are bound forms a ring;
[0011]each L″ is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5, or a bond, or together with R2and the atom to which both R2and L″ are bound forms a ring;
[0012]each R1is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, each being optionally substituted with halo-, or together with L′ and the atom to which both R1and L′ are bound forms a ring;
[0013]each R2is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, each being optionally substituted with halo-, or together with L″ and the atom to which both R2and L″ are bound forms a ring;
[0014]each R3is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, cycloalkylalkyl, aryl, or heteroaryl, each being optionally substituted with R5;
[0015]each L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, heteroarylene, or [—R4—K—R4-]n, each being optionally substituted with R5;
[0016]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene;
[0017]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0018]each n is independently 1, 2, 3, 4, or 5;
[0019]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0020]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0021]each R7is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with a D residue;
[0022]each R8is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with an E residue;
[0023]each v and w is independently an integer from 1-1000;
[0025]u is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; and
[0026]each x, y and z is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, or
[0000]a pharmaceutically-acceptable salt thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
[0027]FIG. 1 illustrates cell viability over time after treatment with a peptidomimetic macrocycle.
[0028]FIG. 2 illustrates cell viability over time after treatment with a peptidomimetic macrocycle.
[0029]FIG. 3 illustrates cell viability over time after treatment with a peptidomimetic macrocycle.
[0030]FIG. 4 illustrates cell viability over time after treatment with a peptidomimetic macrocycle.
[0031]FIG. 5 illustrates normalized fluorescence resonance energy transfer (FRET) signal after treatment with vehicle, a peptidomimetic macrocycle, or a BH3 mimetic.
[0032]FIG. 6 illustrates concentration of a peptidomimetic macrocycle in tissue over time after treatment.
[0033]FIG. 7 illustrates percentage remaining of a peptidomimetic macrocycle in plasma over time after treatment.
[0034]FIG. 8 illustrates results after A375-P cells were treated with BIM SAHBA1or Aileron peptide 1 (40 μM).
[0035]FIG. 9 illustrates results after SK-MEL-2 cells were treated with BIM SAHBA1or Aileron peptide 1 (40 μM).
[0036]FIG. 10 illustrates results after SK-MEL-28 cells were treated with BIM SAHBA1or Aileron peptide 1 (40 μM).
[0037]FIG. 11 illustrates results after A375-P cells were treated with Aileron peptide 2 or Aileron peptide 3 (40 μM).
[0038]FIG. 12 illustrates results after SK-MEL-2 cells were treated with Aileron peptide 2 or Aileron peptide 3 (40 μM).
[0039]FIG. 13 illustrates results after SK-MEL-28 cells were treated with Aileron peptide 2 or Aileron peptide 3 (40 μM).
[0040]FIG. 14 illustrates how a stapled peptide derived from BIM broadly targets BCL-2 family proteins, neutralizes BIM's prosurvival relatives, and directly activates BAX.
[0041]FIG. 15 illustrates how a BH3-only protein (BIM) can directly activate mitochondrial BAK and cytosolic BAX, and inhibit the capacity of anti-apoptotic proteins to sequester activate forms of BAK and BAX, leading the inactive monomers of BAK and BAX to transform to toxic pore-forming proteins.
[0042]FIG. 16 compares high resolution X-ray structures of: a stapled BIM peptide bound to MCL-1; Noxa BH3 bound to MCL-1; and BIM BH3 bound to MCL-1.
[0043]FIG. 17 shows a 2 angstrom X-ray structure of a stapled BIM-BH3 peptide bound to MCL-1.
[0044]FIG. 18 illustrates how stapled BIM peptides of the disclosure can disrupt the formation of MCL-1/BAK complexes in living cells.
[0045]FIG. 19 compares normalized FRET signals of samples to determine the samples' effects in disrupting MCL-1/BAK protein-protein interactions.
[0046]FIG. 20 shows that cross-linked peptide #16 exhibited on-mechanism cytotoxic activity against BAX-BAKwt/wtMEF cells but did not exhibit on-mechanism cytotoxic activity in BAX-BAK−/− double knock outs (DKO).
[0047]FIG. 21 shows that treatment of A375-P (1), SK-MEL-2 (2), and SK-MEL-28 (3) with peptide #16 induced higher levels of caspase-3/7 activation than the BIM SAHBA1control.
[0048]FIG. 22 shows that treatment of A375-P (1), SK-MEL-2 (2), and SK-MEL-28 (3) with peptide #16 decreased the % viability of the cells, while treatment with BIM SAHBA1had no effect on % viability.
[0049]FIG. 23 shows that peptide #16 was ten times more potent than BIM SAHBA1in the MCL-1-1 driven Raji cell line.
[0050]FIG. 24 shows that Raji cell proliferation (fraction of control) decreased with increasing doses of peptide #16 in a dose-dependent manner.
[0051]FIG. 25 shows that Raji cell proliferation (fraction of control) decreased with increasing doses of ABT-199 in a dose-dependent manner.
[0052]FIG. 26 shows that the combination index (CI) of the combination study had additive to synergistic complementary effects.
DETAILED DESCRIPTION OF THE INVENTION
[0053]The terminology used herein is for the purpose of describing particular cases only and is not intended to be limiting. As used herein, the singular forms “a”, “an” and “the” are intended to include the plural forms as well, unless the context clearly indicates otherwise. Furthermore, to the extent that the terms “including”, “includes”, “having”, “has”, “with”, or variants thereof are used in either the detailed description or the claims, such terms are intended to be inclusive in a manner similar to the term “comprising”.
[0054]The term “about” or “approximately” can mean within an acceptable error range for the particular value as determined by one of ordinary skill in the art, which will depend in part on how the value is measured or determined, i.e., the limitations of the measurement system. For example, “about” can mean within 1 or more than 1 standard deviation, per the practice in the art. Alternatively, “about” can mean a range of up to 20%, up to 10%, up to 5%, or up to 1% of a given value. Alternatively, particularly with respect to biological systems or processes, the term can mean within an order of magnitude, within 5-fold, and more preferably within 2-fold, of a value. Where particular values are described in the application and claims, unless otherwise stated the term “about” meaning within an acceptable error range for the particular value should be assumed. The term “about” has the meaning as commonly understood by one of ordinary skill in the art. In some embodiments, the term “about” refers to ±10%. In some embodiments, the term “about” refers to ±5%.
[0055]As used herein, the term “macrocycle” refers to a molecule having a chemical structure including a ring or cycle formed by at least 9 covalently bonded atoms.
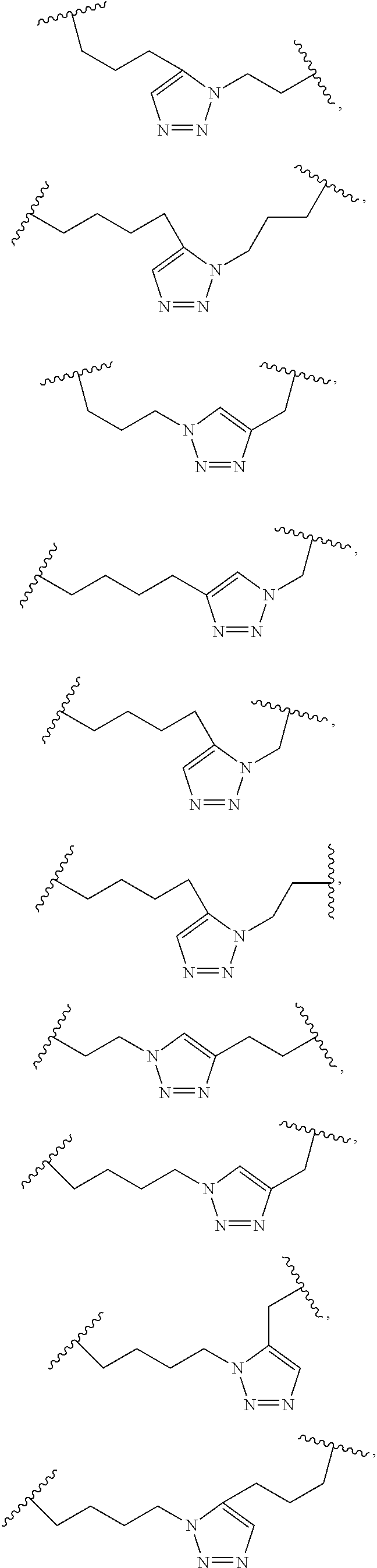
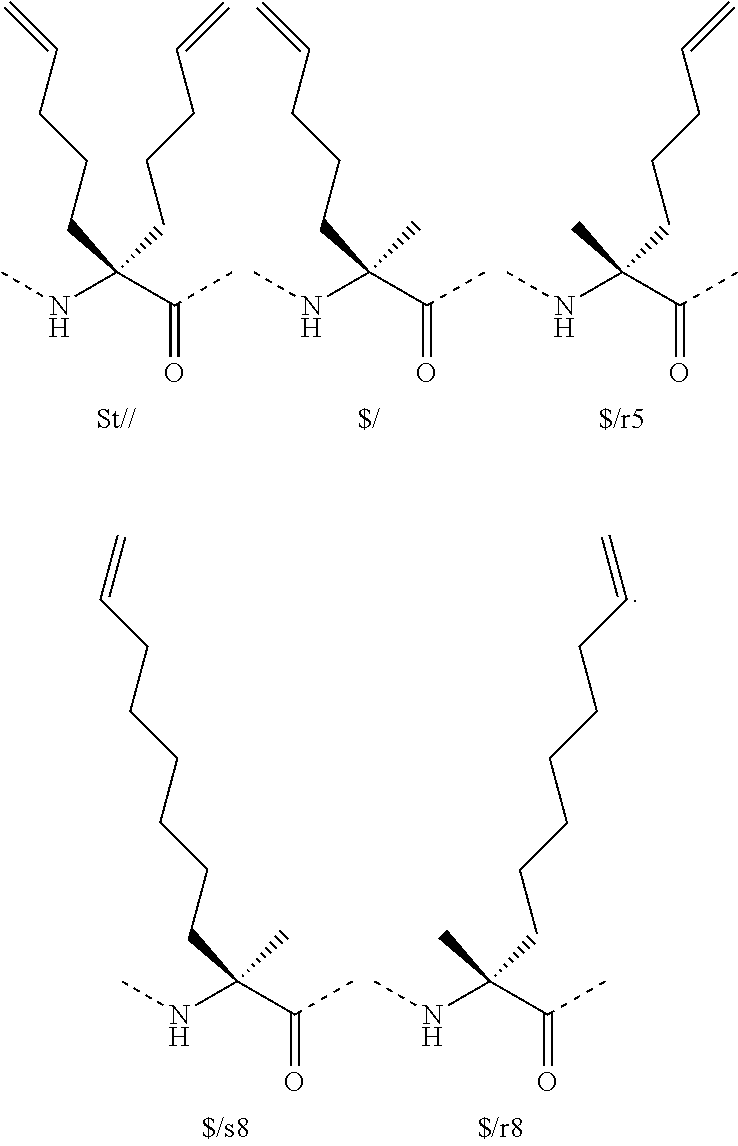
[0056]As used herein, the term “peptidomimetic macrocycle” or “crosslinked polypeptide” refers to a compound comprising a plurality of amino acid residues joined by a plurality of peptide bonds and at least one macrocycle-forming linker which forms a macrocycle between a first naturally-occurring or non-naturally-occurring amino acid residue (or analogue) and a second naturally-occurring or non-naturally-occurring amino acid residue (or analogue) within the same molecule. Peptidomimetic macrocycles include embodiments where the macrocycle-forming linker connects the α carbon of the first amino acid residue (or analogue) to the α carbon of the second amino acid residue (or analogue). The peptidomimetic macrocycles optionally include one or more non-peptide bonds between one or more amino acid residues or amino acid analogue residues, and optionally include one or more non-naturally-occurring amino acid residues or amino acid analogue residues in addition to any which form the macrocycle. A “corresponding uncrosslinked polypeptide” when referred to in the context of a peptidomimetic macrocycle is understood to relate to a polypeptide of the same length as the macrocycle and comprising the equivalent natural amino acids of the wild-type sequence corresponding to the macrocycle.
[0057]As used herein, the term “stability” refers to the maintenance of a defined secondary structure in solution by a peptidomimetic macrocycle of the invention as measured by circular dichroism, NMR or another biophysical measure, or resistance to proteolytic degradation in vitro or in vivo. Non-limiting examples of secondary structures contemplated in this invention are α-helices, 310helices, β-turns, and β-pleated sheets.
[0058]As used herein, the term “helical stability” refers to the maintenance of a helical structure by a peptidomimetic macrocycle of the invention as measured by circular dichroism or NMR. For example, in some embodiments, the peptidomimetic macrocycles of the invention exhibit at least a 1.25, 1.5, 1.75 or 2-fold increase in α-helicity as determined by circular dichroism compared to a corresponding uncrosslinked macrocycle.
[0059]The term “amino acid” refers to a molecule containing both an amino group and a carboxyl group. Suitable amino acids include, without limitation, both the D- and L-isomers of the naturally-occurring amino acids, as well as non-naturally occurring amino acids prepared by organic synthesis or other metabolic routes. The term amino acid, as used herein, includes without limitation, α-amino acids, natural amino acids, non-natural amino acids, and amino acid analogues.
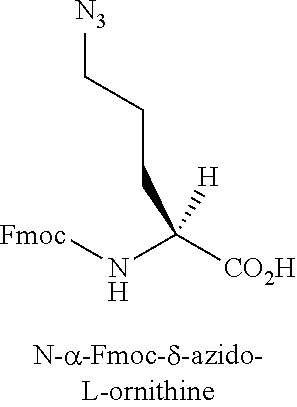
[0060]The term “α-amino acid” refers to a molecule containing both an amino group and a carboxyl group bound to a carbon which is designated the α-carbon.
[0061]The term “β-amino acid” refers to a molecule containing both an amino group and a carboxyl group in a β configuration. The abbreviation “b-” prior to an amino acid represent a beta configuration for the amino acid.
[0062]The term “naturally occurring amino acid” refers to any one of the twenty amino acids commonly found in peptides synthesized in nature, and known by the one letter abbreviations A, R, N, C, D, Q, E, G, H, I, L, K, M, F, P, S, T, W, Y and V.
[0063]The following Table shows a summary of the properties of natural amino acids:
[0000] |
| Alanine | Ala | A | nonpolar | neutral | 1.8 |
| Arginine | Arg | R | polar | positive | −4.5 |
| Asparagine | Asn | N | polar | neutral | −3.5 |
| Aspartie acid | Asp | D | polar | negative | −3.5 |
| Cysteine | Cys | C | polar | neutral | 2.5 |
| Glutamic acid | Glu | E | polar | negative | −3.5 |
| Glutamine | Gln | Q | polar | neutral | −3.5 |
| Glycine | Gly | G | nonpolar | neutral | −0.4 |
| Histidine | His | H | polar | positive(10%) | −3.2 |
| | | | neutral(90%) |
| Isoleucine | Ile | I | nonpolar | neutral | 4.5 |
| Leucine | Leu | L | nonpolar | neutral | 3.8 |
| Lysine | Lys | K | polar | positive | −3.9 |
| Methionine | Met | M | nonpolar | neutral | 1.9 |
| Phenylalanine | Phe | F | nonpolar | neutral | 2.8 |
| Proline | Pro | P | nonpolar | neutral | −1.6 |
| Serine | Ser | S | polar | neutral | −0.8 |
| Threonine | Thr | T | polar | neutral | −0.7 |
| Tryptophan | Trp | W | nonpolar | neutral | −0.9 |
| Tyrosine | Tyr | Y | polar | neutral | −1.3 |
| Valine | Val | V | nonpolar | neutral | 4.2 |
|
[0064]“Hydrophobic amino acids” include small hydrophobic amino acids and large hydrophobic amino acids. “Small hydrophobic amino acids” are glycine, alanine, proline, and analogues thereof. “Large hydrophobic amino acids” are valine, leucine, isoleucine, phenylalanine, methionine, tryptophan, tyrosine, and analogues thereof. “Polar amino acids” are serine, threonine, asparagine, glutamine, cysteine, and analogues thereof. “Charged amino acids” include positively charged amino acids and negatively charged amino acids. “Positively charged amino acids” include lysine, arginine, histidine, and analogues thereof. “Negatively charged amino acids” include aspartate, glutamate, and analogues thereof.
[0065]The term “amino acid analogue” refers to a molecule which is structurally similar to an amino acid and which can be substituted for an amino acid in the formation of a peptidomimetic macrocycle. Amino acid analogues include, without limitation, β-amino acids and amino acids where the amino or carboxy group is substituted by a similarly reactive group (e.g., substitution of the primary amine with a secondary or tertiary amine, or substitution of the carboxy group with an ester).
[0066]The term “non-natural amino acid” refers to an amino acid which is not one of the twenty amino acids commonly found in peptides synthesized in nature, and known by the one letter abbreviations A, R, N, C, D, Q, E, G, H, I, L, K, M, F, P, S, T, W, Y and V. Non-natural amino acids or amino acid analogues include, without limitation, structures according to the following:
[0067]Amino acid analogues include β-amino acid analogues. Examples of β-amino acid analogues include, but are not limited to, the following: cyclic β-amino acid analogues; β-alanine; (R)-β-phenylalanine; (R)-1,2,3,4-tetrahydro-isoquinoline-3-acetic acid; (R)-3-amino-4-(1-naphthyl)-butyric acid; (R)-3-amino-4-(2,4-dichlorophenyl)butyric acid; (R)-3-amino-4-(2-chlorophenyl)-butyric acid; (R)-3-amino-4-(2-cyanophenyl)-butyric acid; (R)-3-amino-4-(2-fluorophenyl)-butyric acid; (R)-3-amino-4-(2-furyl)-butyric acid; (R)-3-amino-4-(2-methylphenyl)-butyric acid; (R)-3-amino-4-(2-naphthyl)-butyric acid; (R)-3-amino-4-(2-thienyl)-butyric acid; (R)-3-amino-4-(2-trifluoromethylphenyl)-butyric acid; (R)-3-amino-4-(3,4-dichlorophenyl)butyric acid; (R)-3-amino-4-(3,4-difluorophenyl)butyric acid; (R)-3-amino-4-(3-benzothienyl)-butyric acid; (R)-3-amino-4-(3-chlorophenyl)-butyric acid; (R)-3-amino-4-(3-cyanophenyl)-butyric acid; (R)-3-amino-4-(3-fluorophenyl)-butyric acid; (R)-3-amino-4-(3-methylphenyl)-butyric acid; (R)-3-amino-4-(3-pyridyl)-butyric acid; (R)-3-amino-4-(3-thienyl)-butyric acid; (R)-3-amino-4-(3-trifluoromethylphenyl)-butyric acid; (R)-3-amino-4-(4-bromophenyl)-butyric acid; (R)-3-amino-4-(4-chlorophenyl)-butyric acid; (R)-3-amino-4-(4-cyanophenyl)-butyric acid; (R)-3-amino-4-(4-fluorophenyl)-butyric acid; (R)-3-amino-4-(4-iodophenyl)-butyric acid; (R)-3-amino-4-(4-methylphenyl)-butyric acid; (R)-3-amino-4-(4-nitrophenyl)-butyric acid; (R)-3-amino-4-(4-pyridyl)-butyric acid; (R)-3-amino-4-(4-trifluoromethylphenyl)-butyric acid; (R)-3-amino-4-pentafluoro-phenylbutyric acid; (R)-3-amino-5-hexenoic acid; (R)-3-amino-5-hexynoic acid; (R)-3-amino-5-phenylpentanoic acid; (R)-3-amino-6-phenyl-5-hexenoic acid; (S)-1,2,3,4-tetrahydro-isoquinoline-3-acetic acid; (S)-3-amino-4-(1-naphthyl)-butyric acid; (S)-3-amino-4-(2,4-dichlorophenyl)butyric acid; (S)-3-amino-4-(2-chlorophenyl)-butyric acid; (S)-3-amino-4-(2-cyanophenyl)-butyric acid; (S)-3-amino-4-(2-fluorophenyl)-butyric acid; (S)-3-amino-4-(2-furyl)-butyric acid; (S)-3-amino-4-(2-methylphenyl)-butyric acid; (S)-3-amino-4-(2-naphthyl)-butyric acid; (S)-3-amino-4-(2-thienyl)-butyric acid; (S)-3-amino-4-(2-trifluoromethylphenyl)-butyric acid; (S)-3-amino-4-(3,4-dichlorophenyl)butyric acid; (S)-3-amino-4-(3,4-difluorophenyl)butyric acid; (S)-3-amino-4-(3-benzothienyl)-butyric acid; (S)-3-amino-4-(3-chlorophenyl)-butyric acid; (S)-3-amino-4-(3-cyanophenyl)-butyric acid; (S)-3-amino-4-(3-fluorophenyl)-butyric acid; (S)-3-amino-4-(3-methylphenyl)-butyric acid; (S)-3-amino-4-(3-pyridyl)-butyric acid; (S)-3-amino-4-(3-thienyl)-butyric acid; (S)-3-amino-4-(3-trifluoromethylphenyl)-butyric acid; (S)-3-amino-4-(4-bromophenyl)-butyric acid; (S)-3-amino-4-(4-chlorophenyl)-butyric acid; (S)-3-amino-4-(4-cyanophenyl)-butyric acid; (S)-3-amino-4-(4-fluorophenyl)-butyric acid; (S)-3-amino-4-(4-iodophenyl)-butyric acid; (S)-3-amino-4-(4-methylphenyl)-butyric acid; (S)-3-amino-4-(4-nitrophenyl)-butyric acid; (S)-3-amino-4-(4-pyridyl)-butyric acid; (S)-3-amino-4-(4-trifluoromethylphenyl)-butyric acid; (S)-3-amino-4-pentafluoro-phenylbutyric acid; (S)-3-amino-5-hexenoic acid; (S)-3-amino-5-hexynoic acid; (S)-3-amino-5-phenylpentanoic acid; (S)-3-amino-6-phenyl-5-hexenoic acid; 1,2,5,6-tetrahydropyridine-3-carboxylic acid; 1,2,5,6-tetrahydropyridine-4-carboxylic acid; 3-amino-3-(2-chlorophenyl)-propionic acid; 3-amino-3-(2-thienyl)-propionic acid; 3-amino-3-(3-bromophenyl)-propionic acid; 3-amino-3-(4-chlorophenyl)-propionic acid; 3-amino-3-(4-methoxyphenyl)-propionic acid; 3-amino-4,4,4-trifluoro-butyric acid; 3-aminoadipic acid; D-β-phenylalanine; β-leucine; L-β-homoalanine; L-β-homoaspartic acid γ-benzyl ester; L-β-homoglutamic acid δ-benzyl ester; L-β-homoisoleucine; L-β-homoleucine; L-β-homomethionine; L-β-homophenylalanine; L-β-homoproline; L-β-homotryptophan; L-β-homovaline; L-Nω-benzyloxycarbonyl-β-homolysine; Nω-L-β-homoarginine; O-benzyl-L-β-homohydroxyproline; O-benzyl-L-β-homoserine; O-benzyl-L-β-homothreonine; O-benzyl-L-β-homotyrosine; γ-trityl-L-β-homoasparagine; (R)-β-phenylalanine; L-β-homoaspartic acid γ-t-butyl ester; L-β-homoglutamic acid δ-t-butyl ester; L-No)-β-homolysine; Nδ-trityl-L-β-homoglutamine; No)-2,2,4,6,7-pentamethyl-dihydrobenzofuran-5-sulfonyl-L-β-homoarginine; O-t-butyl-L-β-homohydroxy-proline; O-t-butyl-L-β-homoserine; O-t-butyl-L-β-homothreonine; O-t-butyl-L-β-homotyrosine; 2-aminocyclopentane carboxylic acid; and 2-aminocyclohexane carboxylic acid.
[0068]Amino acid analogues include analogues of alanine, valine, glycine or leucine. Examples of amino acid analogues of alanine, valine, glycine, and leucine include, but are not limited to, the following: α-methoxyglycine; α-allyl-L-alanine; α-aminoisobutyric acid; α-methyl-leucine; β-(1-naphthyl)-D-alanine; β-(1-naphthyl)-L-alanine; β-(2-naphthyl)-D-alanine; β-(2-naphthyl)-L-alanine; β-(2-pyridyl)-D-alanine; β-(2-pyridyl)-L-alanine; β-(2-thienyl)-D-alanine; β-(2-thienyl)-L-alanine; β-β-benzothienyl)-D-alanine; β-β-benzothienyl)-L-alanine; β-(3-pyridyl)-D-alanine; β-(3-pyridyl)-L-alanine; β-(4-pyridyl)-D-alanine; β-(4-pyridyl)-L-alanine; β-chloro-L-alanine; β-cyano-L-alanin; β-cyclohexyl-D-alanine; β-cyclohexyl-L-alanine; β-cyclopenten-1-yl-alanine; β-cyclopentyl-alanine; β-cyclopropyl-L-Ala-OH.dicyclohexylammonium salt; β-t-butyl-D-alanine; β-t-butyl-L-alanine; γ-aminobutyric acid; L-α,β-diaminopropionic acid; 2,4-dinitro-phenylglycine; 2,5-dihydro-D-phenylglycine; 2-amino-4,4,4-trifluorobutyric acid; 2-fluoro-phenylglycine; 3-amino-4,4,4-trifluoro-butyric acid; 3-fluoro-valine; 4,4,4-trifluoro-valine; 4,5-dehydro-L-leu-OH.dicyclohexylammonium salt; 4-fluoro-D-phenylglycine; 4-fluoro-L-phenylglycine; 4-hydroxy-D-phenylglycine; 5,5,5-trifluoro-leucine; 6-aminohexanoic acid; cyclopentyl-D-Gly-OH.dicyclohexylammonium salt; cyclopentyl-Gly-OH.dicyclohexylammonium salt; D-α,β-diaminopropionic acid; D-α-aminobutyric acid; D-α-t-butylglycine; D-(2-thienyl)glycine; D-(3-thienyl)glycine; D-2-aminocaproic acid; D-2-indanylglycine; D-allylglycine-dicyclohexylammonium salt; D-cyclohexylglycine; D-norvaline; D-phenylglycine; β-aminobutyric acid; β-aminoisobutyric acid; (2-bromophenyl)glycine; (2-methoxyphenyl)glycine; (2-methylphenyl)glycine; (2-thiazoyl)glycine; (2-thienyl)glycine; 2-amino-3-(dimethylamino)-propionic acid; L-α,β-diaminopropionic acid; L-α-aminobutyric acid; L-α-t-butylglycine; L-(3-thienyl)glycine; L-2-amino-3-(dimethylamino)-propionic acid; L-2-aminocaproic acid dicyclohexyl-ammonium salt; L-2-indanylglycine; L-allylglycine-dicyclohexyl ammonium salt; L-cyclohexylglycine; L-phenylglycine; L-propargylglycine; L-norvaline; N-α-aminomethyl-L-alanine; D-α,γ-diaminobutyric acid; L-α,γ-diaminobutyric acid; β-cyclopropyl-L-alanine; (N-β-(2,4-dinitrophenyl))-L-α,β-diaminopropionic acid; (N-β-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl)-D-α,β-diaminopropionic acid; (N-β-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl)-L-α,β-diaminopropionic acid; (N-β-4-methyltrityl)-L-α,β-diaminopropionic acid; (N-β-allyloxycarbonyl)-L-α,β-diaminopropionic acid; (N-γ-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl)-D-α,γ-diaminobutyric acid; (N-γ-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl)-L-α,γ-diaminobutyric acid; (N-γ-4-methyltrityl)-D-α,γ-diaminobutyric acid; (N-γ-4-methyltrityl)-L-α,γ-diaminobutyric acid; (N-γ-allyloxycarbonyl)-L-α,γ-diaminobutyric acid; D-α,γ-diaminobutyric acid; 4,5-dehydro-L-leucine; cyclopentyl-D-Gly-OH; cyclopentyl-Gly-OH; D-allylglycine; D-homocyclohexylalanine; L-1-pyrenylalanine; L-2-aminocaproic acid; L-allylglycine; L-homocyclohexylalanine; and N-(2-hydroxy-4-methoxy-Bzl)-Gly-OH.
[0069]Amino acid analogues include analogues of arginine or lysine. Examples of amino acid analogues of arginine and lysine include, but are not limited to, the following: citrulline; L-2-amino-3-guanidinopropionic acid; L-2-amino-3-ureidopropionic acid; L-citrulline; Lys(Me)2-OH; Lys(N3)—OH; Nδ-benzyloxycarbonyl-L-ornithine; Nω-nitro-D-arginine; Nω-nitro-L-arginine; α-methyl-ornithine; 2,6-diaminoheptanedioic acid; L-ornithine; (Nδ-1-(4,4-dimethyl-2,6-dioxo-cyclohex-1-ylidene)ethyl)-D-ornithine; (Nδ-1-(4,4-dimethyl-2,6-dioxo-cyclohex-1-ylidene)ethyl)-L-ornithine; (Nδ-4-methyltrityl)-D-ornithine; (Nδ-4-methyltrityl)-L-ornithine; D-ornithine; L-ornithine; Arg(Me)(Pbf)-OH; Arg(Me)2-OH (asymmetrical); Arg(Me)2-OH (symmetrical); Lys(ivDde)-OH; Lys(Me)2-OH.HCl; Lys(Me3)-OH chloride; Nω-nitro-D-arginine; and Nω-nitro-L-arginine.
[0070]Amino acid analogues include analogues of aspartic or glutamic acids. Examples of amino acid analogues of aspartic and glutamic acids include, but are not limited to, the following: α-methyl-D-aspartic acid; α-methyl-glutamic acid; α-methyl-L-aspartic acid; γ-methylene-glutamic acid; (N-γ-ethyl)-L-glutamine; [N-α-(4-aminobenzoyl)]-L-glutamic acid; 2,6-diaminopimelic acid; L-α-aminosuberic acid; D-2-aminoadipic acid; D-α-aminosuberic acid; α-aminopimelic acid; iminodiacetic acid; L-2-amino adipic acid; threo-β-methyl-aspartic acid; γ-carboxy-D-glutamic acid γ,γ-di-t-butyl ester; γ-carboxy-L-glutamic acid γ,γ-di-t-butyl ester; Glu(OAll)-OH; L-Asu(OtBu)-OH; and pyroglutamic acid.
[0071]Amino acid analogues include analogues of cysteine and methionine. Examples of amino acid analogues of cysteine and methionine include, but are not limited to, Cys(farnesyl)-OH, Cys(farnesyl)-OMe, α-methyl-methionine, Cys(2-hydroxyethyl)-OH, Cys(3-aminopropyl)-OH, 2-amino-4-(ethylthio)butyric acid, buthionine, buthioninesulfoximine, ethionine, methionine methylsulfonium chloride, selenomethionine, cysteic acid, [2-(4-pyridyl)ethyl]-DL-penicillamine, [2-(4-pyridyl)ethyl]-L-cysteine, 4-methoxybenzyl-D-penicillamine, 4-methoxybenzyl-L-penicillamine, 4-methylbenzyl-D-penicillamine, 4-methylbenzyl-L-penicillamine, benzyl-D-cysteine, benzyl-L-cysteine, benzyl-DL-homocysteine, carbamoyl-L-cysteine, carboxyethyl-L-cysteine, carboxymethyl-L-cysteine, diphenylmethyl-L-cysteine, ethyl-L-cysteine, methyl-L-cysteine, t-butyl-D-cysteine, trityl-L-homocysteine, trityl-D-penicillamine, cystathionine, homocystine, L-homocystine, (2-aminoethyl)-L-cysteine, seleno-L-cystine, cystathionine, Cys(StBu)-OH, and acetamidomethyl-D-penicillamine.
[0072]Amino acid analogues include analogues of phenylalanine and tyrosine. Examples of amino acid analogues of phenylalanine and tyrosine include β-methyl-phenylalanine, β-hydroxyphenylalanine, α-methyl-3-methoxy-DL-phenylalanine, α-methyl-D-phenylalanine, α-methyl-L-phenylalanine, 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, 2,4-dichloro-phenylalanine, 2-(trifluoromethyl)-D-phenylalanine, 2-(trifluoromethyl)-L-phenylalanine, 2-bromo-D-phenylalanine, 2-bromo-L-phenylalanine, 2-chloro-D-phenylalanine, 2-chloro-L-phenylalanine, 2-cyano-D-phenylalanine, 2-cyano-L-phenylalanine, 2-fluoro-D-phenylalanine, 2-fluoro-L-phenylalanine, 2-methyl-D-phenylalanine, 2-methyl-L-phenylalanine, 2-nitro-D-phenylalanine, 2-nitro-L-phenylalanine, 2;4;5-trihydroxy-phenylalanine, 3,4,5-trifluoro-D-phenylalanine, 3,4,5-trifluoro-L-phenylalanine, 3,4-dichloro-D-phenylalanine, 3,4-dichloro-L-phenylalanine, 3,4-difluoro-D-phenylalanine, 3,4-difluoro-L-phenylalanine, 3,4-dihydroxy-L-phenylalanine, 3,4-dimethoxy-L-phenylalanine, 3,5,3′-triiodo-L-thyronine, 3,5-diiodo-D-tyrosine, 3,5-diiodo-L-tyrosine, 3,5-diiodo-L-thyronine, 3-(trifluoromethyl)-D-phenylalanine, 3-(trifluoromethyl)-L-phenylalanine, 3-amino-L-tyrosine, 3-bromo-D-phenylalanine, 3-bromo-L-phenylalanine, 3-chloro-D-phenylalanine, 3-chloro-L-phenylalanine, 3-chloro-L-tyrosine, 3-cyano-D-phenylalanine, 3-cyano-L-phenylalanine, 3-fluoro-D-phenylalanine, 3-fluoro-L-phenylalanine, 3-fluoro-tyrosine, 3-iodo-D-phenylalanine, 3-iodo-L-phenylalanine, 3-iodo-L-tyrosine, 3-methoxy-L-tyrosine, 3-methyl-D-phenylalanine, 3-methyl-L-phenylalanine, 3-nitro-D-phenylalanine, 3-nitro-L-phenylalanine, 3-nitro-L-tyrosine, 4-(trifluoromethyl)-D-phenylalanine, 4-(trifluoromethyl)-L-phenylalanine, 4-amino-D-phenylalanine, 4-amino-L-phenylalanine, 4-benzoyl-D-phenylalanine, 4-benzoyl-L-phenylalanine, 4-bis(2-chloroethyl)amino-L-phenylalanine, 4-bromo-D-phenylalanine, 4-bromo-L-phenylalanine, 4-chloro-D-phenylalanine, 4-chloro-L-phenylalanine, 4-cyano-D-phenylalanine, 4-cyano-L-phenylalanine, 4-fluoro-D-phenylalanine, 4-fluoro-L-phenylalanine, 4-iodo-D-phenylalanine, 4-iodo-L-phenylalanine, homophenylalanine, thyroxine, 3,3-diphenylalanine, thyronine, ethyl-tyrosine, and methyl-tyrosine.
[0073]Amino acid analogues include analogues of proline. Examples of amino acid analogues of proline include, but are not limited to, 3,4-dehydro-proline, 4-fluoro-proline, cis-4-hydroxy-proline, thiazolidine-2-carboxylic acid, and trans-4-fluoro-proline.
[0074]Amino acid analogues include analogues of serine and threonine. Examples of amino acid analogues of serine and threonine include, but are not limited to, 3-amino-2-hydroxy-5-methylhexanoic acid, 2-amino-3-hydroxy-4-methylpentanoic acid, 2-amino-3-ethoxybutanoic acid, 2-amino-3-methoxybutanoic acid, 4-amino-3-hydroxy-6-methylheptanoic acid, 2-amino-3-benzyloxypropionic acid, 2-amino-3-benzyloxypropionic acid, 2-amino-3-ethoxypropionic acid, 4-amino-3-hydroxybutanoic acid, and α-methylserine.
[0075]Amino acid analogues include analogues of tryptophan. Examples of amino acid analogues of tryptophan include, but are not limited to, the following: α-methyl-tryptophan; β-(3-benzothienyl)-D-alanine; β-(3-benzothienyl)-L-alanine; 1-methyl-tryptophan; 4-methyl-tryptophan; 5-benzyloxy-tryptophan; 5-bromo-tryptophan; 5-chloro-tryptophan; 5-fluoro-tryptophan; 5-hydroxy-tryptophan; 5-hydroxy-L-tryptophan; 5-methoxy-tryptophan; 5-methoxy-L-tryptophan; 5-methyl-tryptophan; 6-bromo-tryptophan; 6-chloro-D-tryptophan; 6-chloro-tryptophan; 6-fluoro-tryptophan; 6-methyl-tryptophan; 7-benzyloxy-tryptophan; 7-bromo-tryptophan; 7-methyl-tryptophan; D-1,2,3,4-tetrahydro-norharman-3-carboxylic acid; 6-methoxy-1,2,3,4-tetrahydronorharman-1-carboxylic acid; 7-azatryptophan; L-1,2,3,4-tetrahydro-norharman-3-carboxylic acid; 5-methoxy-2-methyl-tryptophan; and 6-chloro-L-tryptophan.
[0076]In some embodiments, amino acid analogues are racemic. In some embodiments, the D isomer of the amino acid analogue is used. In some embodiments, the L isomer of the amino acid analogue is used. In other embodiments, the amino acid analogue comprises chiral centers that are in the R or S configuration. In still other embodiments, the amino group(s) of a β-amino acid analogue is substituted with a protecting group, e.g., tert-butyloxycarbonyl (BOC group), 9-fluorenylmethyloxycarbonyl (FMOC), tosyl, and the like. In yet other embodiments, the carboxylic acid functional group of a β-amino acid analogue is protected, e.g., as its ester derivative. In some embodiments the salt of the amino acid analogue is used.
[0077]A “non-essential” amino acid residue is a residue that can be altered from the wild-type sequence of a polypeptide without abolishing or substantially abolishing its essential biological or biochemical activity (e.g., receptor binding or activation). An “essential” amino acid residue is a residue that, when altered from the wild-type sequence of the polypeptide, results in abolishing or substantially abolishing the polypeptide's essential biological or biochemical activity.
[0078]A “conservative amino acid substitution” is one in which the amino acid residue is replaced with an amino acid residue having a similar side chain. Families of amino acid residues having similar side chains have been defined in the art. These families include amino acids with basic side chains (e.g., K, R, H), acidic side chains (e.g., D, E), uncharged polar side chains (e.g., G, N, Q, S, T, Y, C), nonpolar side chains (e.g., A, V, L, I, P, F, M, W), beta-branched side chains (e.g., T, V, I) and aromatic side chains (e.g., Y, F, W, H). Thus, a predicted nonessential amino acid residue in a polypeptide, for example, is replaced with another amino acid residue from the same side chain family. Other examples of acceptable substitutions are substitutions based on isosteric considerations (e.g. norleucine for methionine) or other properties (e.g. 2-thienylalanine for phenylalanine).
[0079]The term “capping group” refers to the chemical moiety occurring at either the carboxy or amino terminus of the polypeptide chain of the subject peptidomimetic macrocycle. The capping group of a carboxy terminus includes an unmodified carboxylic acid (i.e. —COOH) or a carboxylic acid with a substituent. For example, the carboxy terminus can be substituted with an amino group to yield a carboxamide at the C-terminus. Various substituents include but are not limited to primary and secondary amines, including pegylated secondary amines. Non-limiting representative secondary amine capping groups for the C-terminus include:
[0000]

[0080]The capping group of an amino terminus includes an unmodified amine (i.e. —NH2) or an amine with a substituent. For example, the amino terminus can be substituted with an acyl group to yield a carboxamide at the N-terminus. Various substituents include but are not limited to substituted acyl groups, including C1-C6carbonyls, C7-C30carbonyls, and pegylated carbamates. Non-limiting representative capping groups for the N-terminus include:
[0000]

[0081]The term “member” as used herein in conjunction with macrocycles or macrocycle-forming linkers refers to the atoms that form or can form the macrocycle, and excludes substituent or side chain atoms. By analogy, cyclodecane, 1,2-difluoro-decane and 1,3-dimethyl cyclodecane are all considered ten-membered macrocycles as the hydrogen or fluoro substituents or methyl side chains do not participate in forming the macrocycle.
[0082]The symbol “ ” when used as part of a molecular structure refers to a single bond or a trans or cis double bond.
” when used as part of a molecular structure refers to a single bond or a trans or cis double bond.
[0083]The term “amino acid side chain” refers to a moiety attached to the α-carbon (or another backbone atom) in an amino acid. For example, the amino acid side chain for alanine is methyl, the amino acid side chain for phenylalanine is phenylmethyl, the amino acid side chain for cysteine is thiomethyl, the amino acid side chain for aspartate is carboxymethyl, the amino acid side chain for tyrosine is 4-hydroxyphenylmethyl, etc. Other non-naturally occurring amino acid side chains are also included, for example, those that occur in nature (e.g., an amino acid metabolite) or those that are made synthetically (e.g., an α,α di-substituted amino acid).
[0084]The term “α,α di-substituted amino” acid refers to a molecule or moiety containing both an amino group and a carboxyl group bound to a carbon (the α-carbon) that is attached to two natural or non-natural amino acid side chains.
[0085]The term “polypeptide” encompasses two or more naturally or non-naturally-occurring amino acids joined by a covalent bond (e.g., an amide bond). Polypeptides as described herein include full length proteins (e.g., fully processed proteins) as well as shorter amino acid sequences (e.g., fragments of naturally-occurring proteins or synthetic polypeptide fragments).
[0086]The term “macrocyclization reagent” or “macrocycle-forming reagent” as used herein refers to any reagent which may be used to prepare a peptidomimetic macrocycle of the invention by mediating the reaction between two reactive groups. Reactive groups may be, for example, an azide and alkyne, in which case macrocyclization reagents include, without limitation, Cu reagents such as reagents which provide a reactive Cu(I) species, such as CuBr, CuI or CuOTf, as well as Cu(II) salts such as Cu(CO2CH3)2, CuSO4, and CuCl2that can be converted in situ to an active Cu(I) reagent by the addition of a reducing agent such as ascorbic acid or sodium ascorbate. Macrocyclization reagents may additionally include, for example, Ru reagents known in the art such as Cp*RuCl(PPh3)2, [Cp*RuCl]4or other Ru reagents which may provide a reactive Ru(II) species. In other cases, the reactive groups are terminal olefins. In such embodiments, the macrocyclization reagents or macrocycle-forming reagents are metathesis catalysts including, but not limited to, stabilized, late transition metal carbene complex catalysts such as Group VIII transition metal carbene catalysts. For example, such catalysts are Ru and Os metal centers having a +2 oxidation state, an electron count of 16 and pentacoordinated. In other examples, catalysts have W or Mo centers. Various catalysts are disclosed in Grubbs et al., “Ring Closing Metathesis and Related Processes in Organic Synthesis” Acc. Chem. Res. 1995, 28, 446-452, and U.S. Pat. No. 5,811,515; U.S. Pat. No. 7,932,397; U.S. Application No. 2011/0065915; U.S. Application No. 2011/0245477; Yu et al., “Synthesis of Macrocyclic Natural Products by Catalyst-Controlled Stereoselective Ring-Closing Metathesis,” Nature 2011, 479, 88; and Peryshkov et al., “Z-Selective Olefin Metathesis Reactions Promoted by Tungsten Oxo Alkylidene Complexes,” J. Am. Chem. Soc. 2011, 133, 20754. In yet other cases, the reactive groups are thiol groups. In such embodiments, the macrocyclization reagent is, for example, a linker functionalized with two thiol-reactive groups such as halogen groups.
[0087]The term “halo” or “halogen” refers to fluorine, chlorine, bromine or iodine or a radical thereof.
[0088]The term “alkyl” refers to a hydrocarbon chain that is a straight chain or branched chain, containing the indicated number of carbon atoms. For example, C1-C10indicates that the group has from 1 to 10 (inclusive) carbon atoms in it. In the absence of any numerical designation, “alkyl” is a chain (straight or branched) having 1 to 20 (inclusive) carbon atoms in it.
[0089]The term “alkylene” refers to a divalent alkyl (i.e., —R—).
[0090]The term “alkenyl” refers to a hydrocarbon chain that is a straight chain or branched chain having one or more carbon-carbon double bonds. The alkenyl moiety contains the indicated number of carbon atoms. For example, C2-C10indicates that the group has from 2 to 10 (inclusive) carbon atoms in it. The term “lower alkenyl” refers to a C2-C6alkenyl chain. In the absence of any numerical designation, “alkenyl” is a chain (straight or branched) having 2 to 20 (inclusive) carbon atoms in it.
[0091]The term “alkynyl” refers to a hydrocarbon chain that is a straight chain or branched chain having one or more carbon-carbon triple bonds. The alkynyl moiety contains the indicated number of carbon atoms. For example, C2-C10indicates that the group has from 2 to 10 (inclusive) carbon atoms in it. The term “lower alkynyl” refers to a C2-C6alkynyl chain. In the absence of any numerical designation, “alkynyl” is a chain (straight or branched) having 2 to 20 (inclusive) carbon atoms in it.
[0092]The term “aryl” refers to a monocyclic or bicyclic aromatic ring system wherein 0, 1, 2, 3, or 4 atoms of each ring are substituted by a substituent. Examples of aryl groups include phenyl, biphenyl, naphthyl and the like. The term “arylalkoxy” refers to an alkoxy substituted with aryl.
[0093]“Arylalkyl” refers to an aryl group, as defined above, wherein one of the aryl group's hydrogen atoms has been replaced with a C1-C5alkyl group, as defined above. Representative examples of an arylalkyl group include, but are not limited to, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-ethylphenyl, 3-ethylphenyl, 4-ethylphenyl, 2-propylphenyl, 3-propylphenyl, 4-propylphenyl, 2-butylphenyl, 3-butylphenyl, 4-butylphenyl, 2-pentylphenyl, 3-pentylphenyl, 4-pentylphenyl, 2-isopropylphenyl, 3-isopropylphenyl, 4-isopropylphenyl, 2-isobutylphenyl, 3-isobutylphenyl, 4-isobutylphenyl, 2-sec-butylphenyl, 3-sec-butylphenyl, 4-sec-butylphenyl, 2-t-butylphenyl, 3-t-butylphenyl and 4-t-butylphenyl.
[0094]“Arylamido” refers to an aryl group, as defined above, wherein one of the aryl group's hydrogen atoms has been replaced with one or more —C(O)NH2groups. Representative examples of an arylamido group include 2-C(O)NH2-phenyl, 3-C(O)NH2-phenyl, 4-C(O)NH2-phenyl, 2-C(O)NH2-pyridyl, 3-C(O)NH2-pyridyl, and 4-C(O)NH2-pyridyl,
[0095]“Alkylheterocycle” refers to a C1-C5alkyl group, as defined above, wherein one of the C1-C5alkyl group's hydrogen atoms has been replaced with a heterocycle. Representative examples of an alkylheterocycle group include, but are not limited to, —CH2CH2-morpholine, —CH2CH2-piperidine, —CH2CH2CH2-morpholine, and —CH2CH2CH2-imidazole.
[0096]“Alkylamido” refers to a C1-C5alkyl group, as defined above, wherein one of the C1-C5alkyl group's hydrogen atoms has been replaced with a —C(O)NH2group. Representative examples of an alkylamido group include, but are not limited to, —CH2—C(O)NH2, —CH2CH2—C(O)NH2, —CH2CH2CH2C(O)NH2, —CH2CH2CH2CH2C(O)NH2, —CH2CH2CH2CH2CH2C(O)NH2, —CH2CH(C(O)NH2)CH3, —CH2CH(C(O)NH2)CH2CH3, —CH(C(O)NH2)CH2CH3, —C(CH3)2CH2C(O)NH2, —CH2—CH2—NH—C(O)—CH3, —CH2—CH2—NH—C(O)—CH3—CH3, and —CH2—CH2—NH—C(O)—CH═CH2.
[0097]“Alkanol” refers to a C1-C5alkyl group, as defined above, wherein one of the C1-C5alkyl group's hydrogen atoms has been replaced with a hydroxyl group. Representative examples of an alkanol group include, but are not limited to, —CH2OH, —CH2CH2OH, —CH2CH2CH2OH, —CH2CH2CH2CH2OH, —CH2CH2CH2CH2CH2OH, —CH2CH(OH)CH3, —CH2CH(OH)CH2CH3, —CH(OH)CH3and —C(CH3)2CH2OH.
[0098]“Alkylcarboxy” refers to a C1-C5alkyl group, as defined above, wherein one of the C1-C5alkyl group's hydrogen atoms has been replaced with a —COOH group. Representative examples of an alkylcarboxy group include, but are not limited to, —CH2COOH, —CH2CH2COOH, —CH2CH2CH2COOH, —CH2CH2CH2CH2COOH, —CH2CH(COOH)CH3, —CH2CH2CH2CH2CH2COOH, —CH2CH(COOH)CH2CH3, —CH(COOH)CH2CH3and —C(CH3)2CH2COOH.
[0099]The term “cycloalkyl” as employed herein includes saturated and partially unsaturated cyclic hydrocarbon groups having 3 to 12 carbons, preferably 3 to 8 carbons, and more preferably 3 to 6 carbons, wherein the cycloalkyl group additionally is optionally substituted. Some cycloalkyl groups include, without limitation, cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cycloheptyl, and cyclooctyl.
[0100]The term “heteroaryl” refers to an aromatic 5-8 membered monocyclic, 8-12 membered bicyclic, or 11-14 membered tricyclic ring system having 1-3 heteroatoms if monocyclic, 1-6 heteroatoms if bicyclic, or 1-9 heteroatoms if tricyclic, said heteroatoms selected from O, N, or S (e.g., carbon atoms and 1-3, 1-6, or 1-9 heteroatoms of O, N, or S if monocyclic, bicyclic, or tricyclic, respectively), wherein 0, 1, 2, 3, or 4 atoms of each ring are substituted by a substituent. Examples of heteroaryl groups include pyridyl, furyl or furanyl, imidazolyl, benzimidazolyl, pyrimidinyl, thiophenyl or thienyl, quinolinyl, indolyl, thiazolyl, and the like.
[0101]The term “heteroarylalkyl” or the term “heteroaralkyl” refers to an alkyl substituted with a heteroaryl. The term “heteroarylalkoxy” refers to an alkoxy substituted with heteroaryl.
[0102]The term “heteroarylalkyl” or the term “heteroaralkyl” refers to an alkyl substituted with a heteroaryl. The term “heteroarylalkoxy” refers to an alkoxy substituted with heteroaryl.
[0103]The term “heterocyclyl” refers to a nonaromatic 5-8 membered monocyclic, 8-12 membered bicyclic, or 11-14 membered tricyclic ring system having 1-3 heteroatoms if monocyclic, 1-6 heteroatoms if bicyclic, or 1-9 heteroatoms if tricyclic, said heteroatoms selected from O, N, or S (e.g., carbon atoms and 1-3, 1-6, or 1-9 heteroatoms of O, N, or S if monocyclic, bicyclic, or tricyclic, respectively), wherein 0, 1, 2 or 3 atoms of each ring are substituted by a substituent. Examples of heterocyclyl groups include piperazinyl, pyrrolidinyl, dioxanyl, morpholinyl, tetrahydrofuranyl, and the like.
[0104]The term “substituent” refers to a group replacing a second atom or group such as a hydrogen atom on any molecule, compound or moiety. Suitable substituents include, without limitation, halo, hydroxy, mercapto, oxo, nitro, haloalkyl, alkyl, alkaryl, aryl, aralkyl, alkoxy, thioalkoxy, aryloxy, amino, alkoxycarbonyl, amido, carboxy, alkanesulfonyl, alkylcarbonyl, and cyano groups.
[0105]In some embodiments, the compounds of this invention contain one or more asymmetric centers and thus occur as racemates and racemic mixtures, single enantiomers, individual diastereomers and diastereomeric mixtures. All such isomeric forms of these compounds are included in the present invention unless expressly provided otherwise. In some embodiments, the compounds of this invention are also represented in multiple tautomeric forms, in such instances, the invention includes all tautomeric forms of the compounds described herein (e.g., if alkylation of a ring system results in alkylation at multiple sites, the invention includes all such reaction products). All such isomeric forms of such compounds are included in the present invention unless expressly provided otherwise. All crystal forms of the compounds described herein are included in the present invention unless expressly provided otherwise.
[0106]As used herein, the terms “increase” and “decrease” mean, respectively, to cause a statistically significantly (i.e., p<0.1) increase or decrease of at least 5%.
[0107]As used herein, the recitation of a numerical range for a variable is intended to convey that the variable is equal to any of the values within that range. Thus, for a variable which is inherently discrete, the variable is equal to any integer value within the numerical range, including the end-points of the range. Similarly, for a variable which is inherently continuous, the variable is equal to any real value within the numerical range, including the end-points of the range. As an example, and without limitation, a variable which is described as having values between 0 and 2 takes the values 0, 1 or 2 if the variable is inherently discrete, and takes the values 0.0, 0.1, 0.01, 0.001, or any other real values ≥0 and ≤2 if the variable is inherently continuous.
[0108]As used herein, unless specifically indicated otherwise, the word “or” is used in the inclusive sense of “or” and not the exclusive sense of “either/or.”
[0109]The term “on average” represents the mean value derived from performing at least three independent replicates for each data point.
[0110]The term “biological activity” encompasses structural and functional properties of a macrocycle of the invention. Biological activity is, for example, structural stability, alpha-helicity, affinity for a target, resistance to proteolytic degradation, cell penetrability, intracellular stability, in vivo stability, or any combination thereof.
[0111]The details of one or more particular embodiments of the invention are set forth in the accompanying drawings and the description below. Other features, objects, and advantages of the invention will be apparent from the description and drawings, and from the claims.
Peptidomimetic Macrocycles of the Invention
[0112]The present invention provides pharmaceutical formulations comprising an effective amount of peptidomimetic macrocycles or pharmaceutically acceptable salts thereof. The peptidomimetic macrocycles of the invention are cross-linked (e.g., stapled or stitched) and possess improved pharmaceutical properties relative to their corresponding uncross-linked peptidomimetic macrocycles. These improved properties include improved bioavailability, enhanced chemical and in vivo stability, increased potency, and reduced immunogenicity (i.e., fewer or less severe injection site reactions).
[0113]In some embodiments, the peptidomimetic macrocycles of the invention are crosslinked and comprise a warhead, and are used for ligand-directed covalent modification of cysteine- and lysine-containing proteins.
[0114]In some embodiments, the peptide sequences are derived from BIM.
[0115]In some embodiments, a peptidomimetic macrocycle peptide derived from a human BIM peptide can be a peptide comprising 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, or 22 amino acids from a BIM peptide sequence.
[0116]In some embodiments, a peptidomimetic macrocycle peptide derived from a human BIM peptide sequence can be a peptide comprising 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, or 22 amino acids that are different from the selected sequences from which the peptide is derived. In some embodiments, a peptidomimetic macrocycle peptide derived from a human BIM peptide sequence can be a peptide comprising a mutation at amino acid position 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, or 22. In some embodiments, mutations are mutations of non-essential amino acids. In some embodiments, mutations are mutations of essential amino acids. In some embodiments, mutations are mutations of hydrophobic amino acids. In some embodiments, mutations are mutations of naturally occurring amino acids. In some embodiments, mutations are mutations to a conservative amino acid. In some embodiments, a peptidomimetic macrocycle peptide derived from a human BIM peptide sequence can be a peptide comprising 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, or 22 amino acid analogues. In some embodiments, a peptidomimetic macrocycle peptide derived from a human BIM peptide sequence can be a peptide comprising 1 or 2 capping groups.
[0117]In some embodiments, the peptidomimetic macrocycle comprises a C-terminal truncation of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 or 30 amino acids from an amino acid sequence in Table 1. In some embodiments, the peptidomimetic macrocycle comprises a N-terminal truncation of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, or 22 amino acids from the sequence of BIM.
[0118]A non-limiting list of suitable BIM macrocycles for use in the present disclosure are given in Table 1. In Table 1, at the C-terminus, some peptides possess a carboxamide terminus (shown as —NH2); some peptides possess a hydroxyl terminus (shown as —OH); some peptides possess a 5-carboxyfluorescein terminus (shown as −5-FAM); some peptides possess a isobutylamide terminus (shown as —NHiBu); some peptides possess a cyclohexylamide terminus (shown as —NHChx); some peptides possess a cyclohexylmethylamide terminus (shown as —NHMeChx); some peptides possess a phenethylamide terminus (shown as —NHPe); some peptides possess a n-butylamide terminus (shown as —NHBu); some peptides possess a sec-butylamide terminus (shown as —NHsBu); and some peptides possess an uncapped terminus (shown as no terminal modification).
[0119]In Table 1, at the N-terminus, some peptides possess an acetyl terminus (shown as Ac—); some peptides possess a fluorescein isothiocyanate terminus (shown as FITC-); some peptides possess a single-unit polyethylene glycol terminus (shown as dPEG1-); some peptides possess a five-unit polyethylene glycol terminus (shown as dPEG5-); some peptides possess an eleven-unit polyethylene glycol terminus (shown as dPEG11-); some peptides possess a propyl terminus (shown as Pr—); some peptides possess a biotin terminus (shown as Biotin-); some peptides possess a KLH terminus (shown as KLH-); some peptides possess an ovalbumin terminus (shown as OVA-); some peptides possess an uncapped terminus (shown as H—); some peptides possess a isobutyl terminus (shown as iBu-); some peptides possess a decanoyl terminus (shown as Decac-); some peptides possess a benzyl terminus (shown as Bz-); some peptides possess a cyclohexyl terminus (shown as Chx-); some peptides possess a benzyl terminus (shown as Bz-); some peptides possess a Vrl terminus (shown as Vrl-); some peptides possess a HBS terminus (shown as HBS—); some peptides possess a MeIm terminus (shown as MeImC-); some peptides possess a tert-butyl terminus (shown as t-Bu-U—); some peptides possess a nonanoyl terminus (shown as non-U—); some peptides possess a ethyl terminus (shown as Et-U—); some peptides possess a cyclohexyl terminus (shown as Chx-U—); some peptides possess a isopropyl terminus (shown as iPr-U—); some peptides possess a phenyl terminus (shown as Ph-U—); some peptides possess a uric terminus (shown as NH2CO—); some peptides possess a palmitoyl terminus (shown as Pam-); some peptides possess a heptenoic terminus (shown as Hep-); and some peptides possess a 5-carboxytetramethylrhodamine terminus (shown as 5-TAMRA-).
[0000] |
| 1 | Ac-IWIAQELRRIGDEFNAYYARR-NH2 |
|
| 2 | Ac-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 3 | Ac-IWIAQELR$IED$FNAYYARR-NH2 |
|
| 4 | FITC-IWIAQELRRIGDEFNAYYARR-NH2 |
|
| 5 | FITC-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 6 | FITC-IWIAQELR$IED$FNAYYARR-NH2 |
|
| 7 | Ac-IWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 8 | Ac-RWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 9 | Ac-IRIAQQLR$IGD$FNAYYARR-NH2 |
|
| 10 | Ac-RRIAQQLR$IGD$FNAYYARR-NH2 |
|
| 11 | Ac-EIWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 12 | Ac-ERRIAQQLR$IGD$FNAYYARR-NH2 |
|
| 13 | Ac-IRIAQELR$IGD$FNAYYARR-NH2 |
|
| 14 | Ac-RWIAQELR$IGD$FNAYYARR-NH2 |
|
| 15 | Ac-RRIAQELR$IGD$FNAYYARR-NH2 |
|
| 16 | Ac-EIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 17 | Ac-ERWIAQELR$IGD$FNAYYARR-NH2 |
|
| 18 | Ac-EIRIAQELR$IGD$FNAYYARR-NH2 |
|
| 19 | Ac-ERRIAQELR$IGD$FNAYYARR-NH2 |
|
| 20 | PEG1-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 21 | PEG5-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 22 | PEG11-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 23 | Ac-IWIAQELR$IGD$FNASYARR-NH2 |
|
| 24 | Ac-RRIAQELR$IGD$FNASYARR-NH2 |
|
| 25 | Ac-ERRIAQELR$IGD$FNASYARR-NH2 |
|
| 26 | Ac-RRIAQELR$IGD$FNAYYAR-NH2 |
|
| 27 | Ac-RRIAQELR$IGD$FNAYYA-NH2 |
|
| 28 | Ac-RRIAQELR$IGD$FNAYYAib-NH2 |
|
| 29 | Ac-RRIAQELR$IGD$FNASYAib-NH2 |
|
| 30 | Ac-IWIAQELR$IAibD$FNAYYAR-NH2 |
|
| 31 | Ac-IWIAQELR%IAibD%FNAYYAR-NH2 |
|
| 32 | Ac-IRIAQELRRIGDEFNETYTRR-NH2 |
|
| 33 | Ac-IRIAQELR$IGD$FNETYTRR-NH2 |
|
| 34 | Ac-IRIAQELR$IED$FNETYTRR-NH2 |
|
| 35 | Ac-IWIAQELR$/IGD$/FNAYYARR-NH2 |
|
| 36 | Pr-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 37 | Ac-IWIAQELR$IAibD$FNAYYARR-NH2 |
|
| 38 | Ac-IWIAQELR%IAibD%FNAYYARR-NH2 |
|
| 39 | Ac-IWIAQELR$IGD$ANAYYARR-NH2 |
|
| 40 | Ac-IWIAQELR$IGD$FAAYYARR-NH2 |
|
| 41 | Ac-IWIAQELR$IGD$AAAYYARR-NH2 |
|
| 42 | Ac-IWIAQELR%IGD%FNAYYARR-NH2 |
|
| 43 | Ac-AWIAQELR$IGD$FNAYYARR-NH2 |
|
| 44 | Ac-IWAAQELR$IGD$FNAYYARR-NH2 |
|
| 45 | Ac-AWAAQELR$IGD$FNAYYARR-NH2 |
|
| 46 | Ac-IWIAibQELR$IGD$FNAYYARR-NH2 |
|
| 47 | Ac-IWIAQELR$IGD$FNAAYARR-NH2 |
|
| 48 | Ac-IWIAQELR$IGD$FNAYAARR-NH2 |
|
| 49 | Ac-IWIAQELR$IGD$FNAAAARR-NH2 |
|
| 50 | Ac-IWIAQELR$IGD$FNAYYAibRR-NH2 |
|
| 51 | Ac-IAIAQELR%IAibD%FNAYYARR-NH2 |
|
| 52 | Ac-IAIAQELR$IAibD$FNAYYARR-NH2 |
|
| 53 | Ac-DIIRNIAibRHLA$VGD$NleDRSI-NH2 |
|
| 54 | Ac-DIIRNIARHLA$VGD$NleDKSI-NH2 |
|
| 55 | Ac-DIIKNIARHLA$VGD$NleDRSI-NH2 |
|
| 56 | Ac-DIIRNIARHLACVGDCNleDRSI-NH2 |
|
| 57 | Ac-DIIRNIARHLACVAibDCNleDRSI-NH2 |
|
| 58 | Ac-IWIAQELR$IGD$FNA-NH2 |
|
| 59 | Ac-IWIAQELR$IGD$FNRSI-NH2 |
|
| 60 | Ac-IWIAQELR$IGD$FNRSIARR-NH2 |
|
| 61 | Ac-IWIAQELR$IGD$NleDRSI-NH2 |
|
| 62 | Ac-IWIAQELR$VGD$NleDRSI-NH2 |
|
| 63 | Ac-IWIAQEAR$IGA$FNAYYARR-NH2 |
|
| 64 | Ac-WIAQELR$IGD$FNAYYARR-NH2 |
|
| 65 | Ac-IAQELR$IGD$FNAYYARR-NH2 |
|
| 66 | Ac-AQELR$IGD$FNAYYARR-NH2 |
|
| 67 | Ac-QELR$IGD$FNAYYARR-NH2 |
|
| 68 | Ac-ELR$IGD$FNAYYARR-NH2 |
|
| 69 | Ac-IWIAQELR$IGD$FNAYYAR-NH2 |
|
| 70 | Ac-IWIAQELR$IGD$FNAYYA-NH2 |
|
| 71 | Ac-IWIAQELR$IGD$FNAYY-NH2 |
|
| 72 | Ac-IWIAQELR$IGD$FNAY-NH2 |
|
| 73 | Ac-IAIAQELR$IGD$FNAYYARR-NH2 |
|
| 74 | Ac-IWIAAELR$IGD$FNAYYARR-NH2 |
|
| 75 | Ac-IWIAQALR$IGD$FNAYYARR-NH2 |
|
| 76 | Ac-IWIAQEAR$IGD$FNAYYARR-NH2 |
|
| 77 | Ac-IWIAQELA$IGD$FNAYYARR-NH2 |
|
| 78 | Ac-IWIAQELR$AGD$FNAYYARR-NH2 |
|
| 79 | Ac-IWIAQELR$IAD$FNAYYARR-NH2 |
|
| 80 | Ac-IWIAQELR$IGA$FNAYYARR-NH2 |
|
| 81 | Ac-IWIAQELR$IGD$FNAYYAAR-NH2 |
|
| 82 | Ac-IWIAQELR$IGD$FNAYYARA-NH2 |
|
| 83 | Pr-RNIARHLA$VGD$FNAYYARR-NH2 |
|
| 84 | Pr-RNIARHLAib$VGD$FNAYYARR-NH2 |
|
| 85 | Pr-RNIAibRHLAib$VGD$FNAYYARR-NH2 |
|
| 86 | Pr-RNChgARHLA$VAibD$FNAYYARR-NH2 |
|
| 87 | Pr-RNChaARHLA$VAibD$FNAYYARR-NH2 |
|
| 88 | FITC-BaIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 89 | Biotin-AhxIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 90 | KLH-CBaIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 91 | OVA-CBaIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 92 | FITC-BaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 93 | Biotin-AhxIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 94 | KLH-CBaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 95 | OVA-CBaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 96 | FITC-BaIWIAQELR$IED$FNAYYARR-NH2 |
|
| 97 | Biotin-AhxIWIAQELR$IED$FNAYYARR-NH2 |
|
| 98 | FITC-BaIWIAQELR$/IGD$/FNAYYARR-NH2 |
|
| 99 | Ac-BaIWIAQELR$IGD$FNAYYAR-NH2 |
|
| 100 | Ac-IWIAQELR%IGD%FNAYYARR-NH2 |
|
| 101 | H-CBaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 102 | Ac-IWIAQALR$IGD$FAAYYARR-NH2 |
|
| 103 | Ac-IWIAQALR$IAibD$FNAYYARR-NH2 |
|
| 104 | Ac-IWIAQ$LRR$GDEFNAYYARR-NH2 |
|
| 105 | Ac-IWIAQ$LRR$GDAFNAYYARR-NH2 |
|
| 106 | Ac-IWIAQ$LRA$GDAFNAYYARR-NH2 |
|
| 107 | Ac-IWI$QEL$RIGDEFNAYYARR-NH2 |
|
| 108 | Ac-IWI$QAL$RIGDEFNAYYARR-NH2 |
|
| 109 | Ac-IWI$QEL$RIGDAFNAYYARR-NH2 |
|
| 110 | Ac-IWI$QAL$RIGDAFNAYYARR-NH2 |
|
| 111 | Ac-IWIAQALR$IGD$ANAYYARR-NH2 |
|
| 112 | Ac-RWIAQALR$IGD$FNAYYARR-NH2 |
|
| 113 | Ac-RNIAQELR$IGD$FNAYYARR-NH2 |
|
| 114 | Ac-RNIAQALR$IGD$FNAYYARR-NH2 |
|
| 115 | Ac-RRIAQALR$IGD$FNAYYARR-NH2 |
|
| 116 | Ac-RNIAQALR$IGD$ANAYYARR-NH2 |
|
| 117 | Ac-RRIAQALR$IGD$ANAYYARR-NH2 |
|
| 118 | H-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 119 | Ac-IWIAQEChaR$IGD$FNAYYARR-NH2 |
|
| 120 | Ac-IWChgAQELR$IGD$FNAYYARR-NH2 |
|
| 121 | Ac-IRIAQALR$IGD$FNAYYARR-NH2 |
|
| 122 | Ac-IWIAQAibLR$IGD$FNAYYARR-NH2 |
|
| 123 | Ac-IWIAibQALR$IGD$FNAYYARR-NH2 |
|
| 124 | Ac-IWIAQALR$IGD$FNAibYYARR-NH2 |
|
| 125 | Ac-IWIAQALR$IGD$FNAYYAibRR-NH2 |
|
| 126 | Ac-IWIAQALR$IGD$FNASIARR-NH2 |
|
| 127 | Ac-IWIAQALR$IGD$FNAFYARR-NH2 |
|
| 128 | Ac-IWIAQALR$IGD$FNAFFARR-NH2 |
|
| 129 | Ac-IWIAQALR$IGD$FNARRA-NH2 |
|
| 130 | Ac-IWIAQALR$IGD$FNAYKA-NH2 |
|
| 131 | Ac-IWIAQALR$IGD$FNAYK-NH2 |
|
| 132 | Ac-IWIAQALR$IGD$FNASKARR-NH2 |
|
| 133 | Ac-RRIAQQLR$IGD$ANAYYARR-NH2 |
|
| 134 | Ac-WIAQQLR$IGD$FNAYYARR-NH2 |
|
| 135 | Pr-WIAQQLR$IGD$FNAYYARR-NH2 |
|
| 136 | Ac-RWIAQQLR$IGN$FNAYYARR-NH2 |
|
| 137 | H-NMeRWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 138 | Ac-NMeRWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 139 | Ac-IWIAQHLR$IGD$FNAYYARR-NH2 |
|
| 140 | Ac-RWIAQHLR$IGD$FNAYYARR-NH2 |
|
| 141 | Ac-RWIAQELR$ChgGD$FNAYYARR-NH2 |
|
| 142 | Ac-RWIAQELR$ChaGD$FNAYYARR-NH2 |
|
| 143 | Ac-IWIAQQLR$IGD$FNAFFARR-NH2 |
|
| 144 | Ac-RWIAQQLR$IGD$FNAFYARR-NH2 |
|
| 145 | Ac-RWIAQQLR$IGD$FNAYFARR-NH2 |
|
| 146 | Ac-RWIAQQLR$IGD$FNATIARR-NH2 |
|
| 147 | Ac-RWIAQQLR$IGD$FNAYYAR-NH2 |
|
| 148 | Ac-RWIAQQLR$IGD$FNAYYA-NH2 |
|
| 149 | Ac-RWIAQQLR$IGD$FNAYY-NH2 |
|
| 150 | Ac-IWIAQ$LRR$GDQFNAYYARR-NH2 |
|
| 151 | Ac-IWIAQ$LRQ$GDQFNAYYARR-NH2 |
|
| 152 | Ac-RWIAQ$LRA$GDQFNAYYARR-NH2 |
|
| 153 | H-CBaIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 154 | H-CBaIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 155 | H-CBaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 156 | H-CBaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 157 | Ac-RRIAQQLR$IGD$FNAYYAR-NH2 |
|
| 158 | Ac-RRIAQALR$IGD$FNAYYAR-NH2 |
|
| 159 | Ac-RRIAQQLR$IGD$FNAYYA-NH2 |
|
| 160 | Ac-IWIAQQLR$IGD$FNARRA-NH2 |
|
| 161 | Ac-RWIAQQLR$IGD$FNARRA-NH2 |
|
| 162 | Ac-RRIAQQLR$IGD$FNARRA-NH2 |
|
| 163 | Ac-RRIAQQLR$IGD$FNARRA-NH2 |
|
| 164 | Ac-RWIAQQLR$IGD$FNARYA-NH2 |
|
| 165 | Ac-RWIAQQLR$IGD$FNAYRA-NH2 |
|
| 166 | Ac-RWIAQQLR$IGD$FNARYA-NH2 |
|
| 167 | Ac-RWIAQQLR$IGD$FNAYRA-NH2 |
|
| 168 | Ac-RRIAQQLR$IGD$FNASIA-NH2 |
|
| 169 | Ac-RRIAQALR$IGD$FNASIA-NH2 |
|
| 170 | Ac-RRIAQALR$IGD$FNASI-NH2 |
|
| 171 | Ac-RWIAQQLR$IGD$FNARR-NH2 |
|
| 172 | Ac-RWIAQQLR$IGD$FNAR-NH2 |
|
| 173 | Ac-RRIAQQLR$IGD$FNAR-NH2 |
|
| 174 | Ac-RRIAQQLR$IGD$FNAib-NH2 |
|
| 175 | Ac-RRIAQQLR$IGD$FNA-NH2 |
|
| 176 | Ac-RRIAQQLR$IGD$FNARRA-NH2 |
|
| 177 | Ac-RRIAQQLR$IGD$FNAYYA-NH2 |
|
| 178 | Ac-RRIAQQLR$IGD$FNAYYAib-NH2 |
|
| 179 | Ac-RWIAQQLR$IGD$FNAibRRA-NH2 |
|
| 180 | Ac-RWIAibQQLR$IGD$FNARRA-NH2 |
|
| 181 | Ac-RWAibAQQLR$IGD$FNARRA-NH2 |
|
| 182 | Ac-RAibIAQQLR$IGD$FNARRA-NH2 |
|
| 183 | Ac-RFIAQQLR$IGD$FNAYYARR-NH2 |
|
| 184 | Ac-RFIAQQLR$IGD$FNARRA-NH2 |
|
| 185 | Ac-RAibIAQQLR$IGD$FNAYYARR-NH2 |
|
| 186 | Ac-RWIAQQhFR$IGD$FNAYYARR-NH2 |
|
| 187 | Ac-RWIAQQ3cfR$IGD$FNAYYARR-NH2 |
|
| 188 | Ac-RWIAQQ1NalR$IGD$FNAYYARR-NH2 |
|
| 189 | Ac-RWIAQQ2NalR$IGD$FNAYYARR-NH2 |
|
| 190 | Ac-IWIAQEAR$IGD$ANAYYARR-NH2 |
|
| 191 | Ac-RRI$QAL$RIGDAibFNARRA-NH2 |
|
| 192 | Ac-RRIAQ$LRR$GDAibFNARRA-NH2 |
|
| 193 | iBu-RWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 194 | Dec-RWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 195 | Bz-RWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 196 | H-RWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 197 | Chx-RWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 198 | Vrl-RWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 199 | PhAc-RWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 200 | MeImC-RWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 201 | Pr-RWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 202 | Ac-RWIAQALR$IGD$FNASIARR-NH2 |
|
| 203 | Ac-RWIAQQLR$IGD$FNASIARR-NH2 |
|
| 204 | Ac-RWIAQALR$IGD$FNAFYARR-NH2 |
|
| 205 | Ac-RRIAQALR$IGD$FNAFYA-NH2 |
|
| 206 | Ac-RRIAQQLR$IGD$FNAFYA-NH2 |
|
| 207 | Ac-RWIAQALR$IGD$FNAYYARR-NHPr |
|
| 208 | Ac-RWIAQALR$IGD$FNAYYARR-NHiBu |
|
| 209 | Ac-RWIAQALR$IGD$FNAYYARR-NHChx |
|
| 210 | Ac-RWIAQALR$IGD$FNAYYARR-NHBn |
|
| 211 | Ac-RWIAQALR$IGD$FNAYYARR-NHMeChx |
|
| 212 | Ac-RWIAQALR$IGD$FNAYYARR-NHEtPh |
|
| 213 | Ac-RWIAQALR$IGD$FNAYYARR-NHsBu |
|
| 214 | Ac-RWIAQALR$IGD$FNARR-NHPr |
|
| 215 | Ac-RWIAQALR$IGD$FNARR-NHiBu |
|
| 216 | Ac-RWIAQALR$IGD$FNARR-NHChx |
|
| 217 | Ac-RWIAQALR$IGD$FNARR-NHBn |
|
| 218 | Ac-RWIAQALR$IGD$FNARR-NHMeChx |
|
| 219 | Ac-RWIAQALR$IGD$FNARR-NHEtPh |
|
| 220 | Ac-RWIAQALR$IGD$FNARR-NHsBu |
|
| 221 | Ac-RWIAQALR$IGA$FNAYYARR-NH2 |
|
| 222 | Ac-RWIAQALR$IGN$FNAYYARR-NH2 |
|
| 223 | Ac-IWIAQALR$IGA$FNARRA-NH2 |
|
| 224 | Ac-IWIAQALR$IGN$FNARRA-NH2 |
|
| 225 | Ac-RWIAQAFR$IGD$FNAYYARR-NH2 |
|
| 226 | H-CAhxIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 227 | H-CAhxIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 228 | Pr-IPIAQALR$IGD$FNARRA-NH2 |
|
| 229 | Pr-PWIAQALR$IGD$FNARRA-NH2 |
|
| 230 | KLH-CAhxIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 231 | OVA-CAhxIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 232 | KLH-CAhxIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 233 | OVA-CAhxIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 234 | Ac-IWIAEELA$IGD$FDAYYA-NH2 |
|
| 235 | FITC-BaIWIAEELA$IGD$FDAYYA-NH2 |
|
| 236 | Ac-IWIAEELA$IGD$FDAYYAAA-NH2 |
|
| 237 | FITC-BaIWIAEELA$IGD$FDAYYAAA-NH2 |
|
| 238 | Ac-RWIAQALR$IGD$FNAYKARR-NH2 |
|
| 239 | Ac-RWIAQQLR$IGD$FNAYKARR-NH2 |
|
| 240 | Ac-RWIAQALR$IGD$FNAYK-NH2 |
|
| 241 | Ac-RWIAQALR$IGD$FNAFK-NH2 |
|
| 242 | Ac-RWIAQALR$IGD$hFNAYYARR-NH2 |
|
| 243 | Ac-RWIAQALR$IGD$1NalNAYYARR-NH2 |
|
| 244 | Ac-RWIAQALR$IGD$2NalNAYYARR-NH2 |
|
| 245 | Ac-R2NalIAQALR$IGD$FNAYYARR-NH2 |
|
| 246 | Ac-RhFIAQALR$IGD$FNAYYARR-NH2 |
|
| 247 | Ac-RWIAQALR$IGNle$FNAYYARR-NH2 |
|
| 248 | Ac-RWNleAQALR$IGD$FNAYYARR-NH2 |
|
| 249 | Ac-RWIAQNleLR$IGD$FNAYYARR-NH2 |
|
| 250 | Ac-RWIAQQLR$IGD$FNAYK-NH2 |
|
| 251 | H-CAhxIWIAQELR$IED$FNAYYARR-NH2 |
|
| 252 | Ac-IWIAQALR$IGD$FNAYOrnARR-NH2 |
|
| 253 | Ac-IWIAQALR$IGD$FNAYOrn-NH2 |
|
| 254 | Ac-IWIAQALR$IGD$FNAYR-NH2 |
|
| 255 | Ac-IWIAQALR$IGD$FNAYRA-NH2 |
|
| 256 | Ac-IWIAQALR$IFD$FNARRA-NH2 |
|
| 257 | Ac-RWIAQALR$IGD$FNARRA-NH2 |
|
| 258 | Ac-IWIAQELR$ChgGD$FNAYYARR-NH2 |
|
| 259 | Ac-IWIAQQLR$IGD$FNAYY-NH2 |
|
| 260 | Ac-IWIAQ$LRA$GDQFNAYYARR-NH2 |
|
| 261 | Ac-IWIAQALR$IGD$FAibAYK-NH2 |
|
| 262 | Ac-IWIAQALR$IGD$FAibAYYARR-NH2 |
|
| 263 | Ac-IWIAQALR$IGN$FNAFYARR-NH2 |
|
| 264 | Ac-RWIAQALR$IGN$FNAFYARR-NH2 |
|
| 265 | Ac-IWIAQAibLR$IGN$FNAFYARR-NH2 |
|
| 266 | Ac-IWIAQALR$IGN$FNAibFYARR-NH2 |
|
| 267 | Ac-IWIAQAibLR$IGN$FNAibFYARR-NH2 |
|
| 268 | Pr-RNChgARHLA$VAibD$FNAFYARR-NH2 |
|
| 269 | Ac-IWIAQAAR$IGD$FNAYYARR-NH2 |
|
| 270 | Ac-IWIAQAAR$IGD$ANAYYARR-NH2 |
|
| 271 | Ac-IWIAQAAR$IGA$ANAYYARR-NH2 |
|
| 272 | Ac-IWIAQAAR$IEA$ANAYYARR-NH2 |
|
| 273 | Ac-IWIAQALR$DIG$FNAYYARR-NH2 |
|
| 274 | Ac-IWIAQAAR$DIG$ANAYYARR-NH2 |
|
| 275 | Ac-IWIAQALR$IED$FNAYYARR-NH2 |
|
| 276 | Ac-IWIAQALD$IGR$FNAYYARR-NH2 |
|
| 277 | Ac-IWIAQAAD$IGR$ANAYYARR-NH2 |
|
| 278 | Ac-IWIAQAAD$IER$ANAYYARR-NH2 |
|
| 279 | Ac-IWIAQAibLR$IGD$FNAibYYARR-NH2 |
|
| 280 | Ac-IWIAQQLR$IGD$FNAYRA-NH2 |
|
| 281 | Ac-IWI$QAL$RIGDAibFNAYYARR-NH2 |
|
| 282 | t-Bu-U-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 283 | non-U-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 284 | Et-U-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 285 | Chx-U-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 286 | iPr-U-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 287 | Ph-U-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 288 | NH2CO-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 289 | Ac-IWIAQAAR$IGR$ANAYYARR-NH2 |
|
| 290 | Ac-IWIAQAAD$IGD$ANAYYARR-NH2 |
|
| 291 | Ac-IWIAQALD$IGD$FNAYYARR-NH2 |
|
| 292 | Ac-IWIAQALR$IGR$FNAYYARR-NH2 |
|
| 293 | Ac-IWIAQAAR$IGD$ANAYYARR-NH2 |
|
| 294 | Ac-IWIAQAAD$IGR$ANAYYARR-NH2 |
|
| 295 | Ac-IWIAQALD$IGR$FNAYYARR-NH2 |
|
| 296 | Ac-IWIAQALRRIGDEFNAYYARR-NH2 |
|
| 297 | Ac-IWIAQALR$IGN$FNAYYARR-NH2 |
|
| 298 | Ac-IWIAQALR$IGNle$FNAYYARR-NH2 |
|
| 299 | Ac-IWIAQALR$IGA$FNAFYARR-NH2 |
|
| 300 | Ac-IWIAQALR$IGN$FNAFYARR-NH2 |
|
| 301 | Ac-IWIAQALR$IGNle$FNAFYARR-NH2 |
|
| 302 | Ac-RWIAQAFR$IGD$FNAFYARR-NH2 |
|
| 303 | Ac-IWIAQAFR$IGD$FNAFYARR-NH2 |
|
| 304 | Ac-IWIAQAFR$IGN$FNAYYARR-NH2 |
|
| 305 | Ac-IWIAQAFR$IGN$FNAFYARR-NH2 |
|
| 306 | Ac-IWIAQALR$IG$EFNAYYARR-NH2 |
|
| 307 | Ac-IWIAQALRR$GD$FNAYYARR-NH2 |
|
| 308 | Ac-IWIAQALRAibIGAmDEFNAYYARR-NH2 |
|
| 309 | Ac-IWIAQELR#IGD#FNAYYARR-NH2 |
|
| 310 | Ac-IWIAQELR$IGD#FNAYYARR-NH2 |
|
| 311 | Ac-IWIAQELR#IGD$FNAYYARR-NH2 |
|
| 312 | Ac-IWIAQALR$IGD$FNAYYARR-NHiBu |
|
| 313 | Chx-IWIAQALR$IGD$FNAYYARR-NHiBu |
|
| 314 | Chx-U-IWIAQALR$IGD$FNAYYARR-NHiBu |
|
| 315 | FITC-AhxIWIAQALR$IGD$FNAibYYARR-NH2 |
|
| 316 | FITC-AhxIWIAQALR$IGD$FNAFYARR-NH2 |
|
| 317 | FITC-AhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 318 | FITC-AhxRWIAQALR$IGN$FNAYYARR-NH2 |
|
| 319 | FITC-AhxRWIAQALR$IGNle$FNAYYARR-NH2 |
|
| 320 | FITC-AhxIWIAQALR$IGN$FNAYYARR-NH2 |
|
| 321 | FITC-AhxIWIAQALR$IGNle$FNAYYARR-NH2 |
|
| 322 | Ac-IWIAQELRbKIGDbEFNAYYARR-NH2 |
|
| 323 | Ac-IWIAQELRbEIGDbKFNAYYARR-NH2 |
|
| 324 | Ac-IWIAQELRbKIAibDbEFNAYYARR-NH2 |
|
| 325 | Ac-IWIAQELRbEIAibDbKFNAYYARR-NH2 |
|
| 326 | Ac-IWIAQELR#sIGD#sFNAYYARR-NH2 |
|
| 327 | Ac-IWIAQELR#sIAibD#sFNAYYARR-NH2 |
|
| 328 | Ac-IWIAQELR$sIGD$sFNAYYARR-NH2 |
|
| 329 | Ac-IAmWIAQELR$IGD$FNAYYARR-NH2 |
|
| 330 | Ac-IWIAQELR$r5IGD$r5FNAYYARR-NH2 |
|
| 331 | Ac-IWIA$r5ELR$r5IGDEFNAYYARR-NH2 |
|
| 332 | Ac-IWIA$ELR$IGDEFNAYYARR-NH2 |
|
| 333 | Ac-IWIAQ$r8LRRIGD$FNAYYARR-NH2 |
|
| 334 | Ac-I$r8IAQELR$IGDEFNAYYARR-NH2 |
|
| 335 | HepIAQ$LRRIGDEFNAYYARR-NH2 |
|
| 336 | HepIAQ$LR$IGD$FNAYYARR-NH2 |
|
| 337 | HepWIA$ELRRIGDEFNAYYARR-NH2 |
|
| 338 | HepWIA$ELR$IGD$FNAYYARR-NH2 |
|
| 339 | Ac-I$IAQ$LRRIGDEFNAYYARR-NH2 |
|
| 340 | Ac-I$IAQ$LR$IGD$FNAYYARR-NH2 |
|
| 341 | Ac-IWIAQALE$IGD$FNAYYARR-NH2 |
|
| 342 | Ac-IWIAQALR$IGR$ANAYYARR-NH2 |
|
| 343 | Ac-IWIAQAAE$IGR$ANAYYARR-NH2 |
|
| 344 | Ac-IWIAQAAE$IGE$ANAYYARR-NH2 |
|
| 345 | Ac-RWIAQALR$IGR$FNAFYARR-NH2 |
|
| 346 | Ac-RWIAQALE$IGD$FNAFYARR-NH2 |
|
| 347 | Ac-RWIAQAAR$IGR$ANAFYARR-NH2 |
|
| 348 | Ac-RWIAQAAE$IGD$ANAFYARR-NH2 |
|
| 349 | Ac-RWIAQAAD$IGD$ANAFYARR-NH2 |
|
| 350 | Ac-RWIAQAAE$IGR$ANAFYARR-NH2 |
|
| 351 | Ac-RWIAQAAR$IGD$ANAFYARR-NH2 |
|
| 352 | Ac-RWIAQALR$DIG$FNAFYARR-NH2 |
|
| 353 | Ac-RWIAQALR$IGN$ANAYYARR-NH2 |
|
| 354 | Ac-RWIAQAAR$IGN$ANAYYARR-NH2 |
|
| 355 | Ac-RWIAQAAE$IGN$ANAYYARR-NH2 |
|
| 356 | Ac-RWIAQAAE$IGN$ANAYYARR-NH2 |
|
| 357 | Ac-RWIAQAAE$NIG$ANAYYARR-NH2 |
|
| 358 | Ac-RWIAQAAR$NIG$ANAYYARR-NH2 |
|
| 359 | Ac-IWIAQALR$IGN$ANAYYARR-NH2 |
|
| 360 | Ac-IWIAQAAR$IGN$ANAYYARR-NH2 |
|
| 361 | Ac-IWIAQAAE$IGN$ANAYYARR-NH2 |
|
| 362 | Ac-IWIAQAAE$IGN$ANAYYARR-NH2 |
|
| 363 | Ac-IWIAQAAE$NIG$ANAYYARR-NH2 |
|
| 364 | Ac-IWIAQAAR$NIG$ANAYYARR-NH2 |
|
| 365 | Ac-RWIAQALRRIGNEFNAYYARR-NH2 |
|
| 366 | Ac-IWIAQALRRIGNEFNAYYARR-NH2 |
|
| 367 | Ac-RWIAQALR$IEN$FNAYYARR-NH2 |
|
| 368 | Ac-RWIAQALR$IED$FNAFYARR-NH2 |
|
| 369 | Ac-IWIAQALR$IED$FNAFYARR-NH2 |
|
| 370 | Ac-IWIAQELR$IGR$FNAYYARR-NH2 |
|
| 371 | Ac-IWIAQELRbKIGDbDFNAYYARR-NH2 |
|
| 372 | Ac-IWIAQELRbDIGDbKFNAYYARR-NH2 |
|
| 373 | FITC-AhxRWIAQALRRIGDEFNAFYARR-NH2 |
|
| 374 | FITC-AhxRWIAQALRRIGNEFNAYYARR-NH2 |
|
| 375 | FITC-AhxIWIAQALRRIGNEFNAYYARR-NH2 |
|
| 376 | FITC-AhxIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 377 | Ac-RWIAQALR$/IGN$/FNAYYARR-NH2 |
|
| 378 | Ac-IWIAQELR#cIGR#cFNAYYARR-NH2 |
|
| 379 | Ac-IWIAQELRCIGRCFNAYYARR-NH2 |
|
| 380 | FITC-AhxIWIAQAAR$DIG$ANAYYARR-NH2 |
|
| 381 | Ac-IWIAQQLR%IGD%FNAYYARR-NH2 |
|
| 382 | FITC-AhxRNIARHLA$VGD$NleAibRSI-NH2 |
|
| 383 | FITC-AhxIWIAQALR$IGD$FNAYYARR-NH2 |
|
| 384 | Ac-IWIAQELR#c4IGD#c4FNAYYARR-NH2 |
|
| 385 | Ac-IWIAQELR$c4IGD$c4FNAYYARR-NH2 |
|
| 386 | Ac-IWIAQELR#cIGD#cFNAYYARR-NH2 |
|
| 387 | Ac-IWIAQELR$cIGD$cFNAYYARR-NH2 |
|
| 388 | FITC-AhxIWIAQELR#IGD#FNAYYARR-NH2 |
|
| 389 | 5-FAM-AhxIWIAQELR#c4IGD#c4FNAYYARR-NH2 |
|
| 390 | 5-FAM-AhxIWIAQELR$c4IGD$c4INAYYARR-NH2 |
|
| 391 | FITC-AhxIWIAQELR#cIGD#cFNAYYARR-NH2 |
|
| 392 | FITC-AhxIWIAQELR#sIGD#sFNAYYARR-NH2 |
|
| 393 | FITC-AhxIWIAQELR$cIGD$cFNAYYARR-NH2 |
|
| 394 | Ac-IWIAQELR$4n4IGD$4a5FNAYYARR-NH2 |
|
| 395 | Ac-IWIAQELR$4a5IGD$4n4FNAYYARR-NH2 |
|
| 396 | Ac-IWIAQELR$5n3IGD$5a5FNAYYARR-NH2 |
|
| 397 | Ac-IWIAQELR$5a5IGD$5n3FNAYYARR-NH2 |
|
| 398 | Ac-IWIAQELR#5n3IGD#5a5FNAYYARR-NH2 |
|
| 399 | Ac-IWIAQELR#5a5IGD#5n3FNAYYARR-NH2 |
|
| 400 | FITC-AhxIWIAQELR$4n4IGD$4a5FNAYYARR-NH2 |
|
| 401 | FITC-AhxIWIAQELR$4a5IGD$4n4FNAYYARR-NH2 |
|
| 402 | FITC-AhxIWIAQELR$5n3IGD$5a5FNAYYARR-NH2 |
|
| 403 | FITC-AhxIWIAQELR$5a5IGD$5n3FNAYYARR-NH2 |
|
| 404 | FITC-AhxIWIAQELR#5n3IGD#5a5FNAYYARR-NH2 |
|
| 405 | FITC-AhxIWIAQELR#5a5IGD#5n3FNAYYARR-NH2 |
|
| 406 | Ac-IWIAQALR$IEN$FNAYYARR-NH2 |
|
| 407 | Ac-RWIAQALR$/IGD$/FNAFYARR-NH2 |
|
| 408 | Ac-IWIAQALR$/IGN$/FNAYYARR-NH2 |
|
| 409 | Ac-IWIAQALR$/IGD$/FNAYYARR-NH2 |
|
| 410 | Ac-RWIChaQALR$IGD$FNAFYARR-NH2 |
|
| 411 | Ac-RWIAQALR$IChaD$FNAFYARR-NH2 |
|
| 412 | Ac-RWIAQALR$IGD$FNAFYARR-NH2 |
|
| 413 | Ac-RWIAQALR$IGD$FNChaFYARR-NH2 |
|
| 414 | Ac-RWIAQALR$IGD$FNAFYChaRR-NH2 |
|
| 415 | Ac-IWIChaQALR$IGN$FNAYYARR-NH2 |
|
| 416 | Ac-IWIAQALR$IChaN$FNAYYARR-NH2 |
|
| 417 | Ac-IWIAQALR$IGN$FNAYYARR-NH2 |
|
| 418 | Ac-IWIAQALR$IGN$FNChaYYARR-NH2 |
|
| 419 | Ac-IWIAQALR$IGN$FNAYYChaRR-NH2 |
|
| 420 | HepIAQ$LR$IGD$FNAFYARR-NH2 |
|
| 421 | Ac-YGRKKRRQRRRIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 422 | FITC-AhxYGRKKRRQRRRIWIAQELRRIGDEFNAYYARR- |
| NH2 |
|
| 423 | Ac-RWIAQALR$IGD$FNAFYAHR-NH2 |
|
| 424 | Ac-RWIAQALR$IGD$FNAFYARH-NH2 |
|
| 425 | Ac-RWIAQSLR$IGD$FNAFYARR-NH2 |
|
| 426 | Ac-IWIAQELR#4n4IGD#4a5FNAYYARR-NH2 |
|
| 427 | FITC-AhxRWIAQALR$/IGN$/FNAYYARR-NH2 |
|
| 428 | FITC-AhxRWIAQALR$/IGD$/FNAFYARR-NH2 |
|
| 429 | FITC-AhxIWIAQALR$/IGN$/FNAYYARR-NH2 |
|
| 430 | FITC-AhxIWIAQALR$/IGD$/FNAYYARR-NH2 |
|
| 431 | FITC-AhxIWIAQELR$sIGD$sFNAYYARR-NH2 |
|
| 432 | Biotin-AhxRWIAQALRRIGDEFNAFYARR-NH2 |
|
| 433 | Biotin-AhxRWIAQALRRIGNEFNAYYARR-NH2 |
|
| 434 | Biotin-AhxIWIAQALRRIGNEFNAYYARR-NH2 |
|
| 435 | Biotin-AhxIWIAQALRRIGDEFNAYYARR-NH2 |
|
| 436 | FITC-AhxIWIAQALRRIGDEFNAYYARR-NH2 |
|
| 437 | Biotin-AhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 438 | Biotin-AhxRWIAQALR$IGN$FNAYYARR-NH2 |
|
| 439 | Biotin-AhxIWIAQALR$IGN$FNAYYARR-NH2 |
|
| 440 | Biotin-AhxIWIAQALR$IGD$FNAYYARR-NH2 |
|
| 441 | Biotin-AhxIWIAQALR$IGD$FNAFYARR-NH2 |
|
| 442 | 5-FAM-AhxIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 443 | DuIAQDprLRRIGDEFNAYYARR-NH2 |
|
| 444 | DuIAQDprLRRIGDQFNAYYARR-NH2 |
|
| 445 | DuWIADprALRRIGDEFNAYYARR-NH2 |
|
| 446 | DuWIADprALRRIGDQFNAYYARR-NH2 |
|
| 447 | 5-FAM-AhxIWIAQALRRIGDEFNAYYARR-NH2 |
|
| 448 | 5-FAM-AhxIWIAQALR$IGD$FNAYYARR-NH2 |
|
| 449 | 5-FAM-AhxIWIAQAARRDIGEANAYYARR-NH2 |
|
| 450 | 5-FAM-AhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 451 | 5-FAM-AhxIWIAQALRRIGDEFNAFYARR-NH2 |
|
| 452 | Ac-IWIAQEAmLR$IGD$FNAYYARR-NH2 |
|
| 453 | Ac-IWIAQELR$IGD$FNAibYYARR-NH2 |
|
| 454 | Ac-IWIAQELR$IGD$FNAAmfYARR-NH2 |
|
| 455 | Ac-IWIAQELR$IGD$FNAYAmfARR-NH2 |
|
| 456 | Ac-IWIAQELR$IGD$FNAAmyeYARR-NH2 |
|
| 457 | Ac-IWIAQELR$IGD$FNAYAmyeARR-NH2 |
|
| 458 | Ac-IWIAQELR$IGD$FNAYYAAmrR-NH2 |
|
| 459 | Ac-IWIAQELR$IGD$FNAYFARR-NH2 |
|
| 460 | Ac-IWIAQELR$IGD$FNAFYARR-NH2 |
|
| 461 | Ac-RWIAQELR$IGD$FNAFYARR-NH2 |
|
| 462 | Ac-RWIAQALR$IGD$FNAAmfYARR-NH2 |
|
| 463 | Ac-RWIAQALR$IGD$FNAFYAAmrR-NH2 |
|
| 464 | Ac-IWIA$r5ALRStIGD$FNAYYARR-NH2 |
|
| 465 | Ac-IWIA$ALRStIGDEFN$s8YYARR-NH2 |
|
| 466 | Ac-IWIAQALR$r5IGDStFNA$YARR-NH2 |
|
| 467 | 5-FAM-AhxIWIAQELRbKIGDbDFNAYYARR-NH2 |
|
| 468 | 5-FAM-AhxIWIAQELRbDIGDbKFNAYYARR-NH2 |
|
| 469 | 5-FAM-AhxIWIAQELR#IGD#FNAYYARR-NH2 |
|
| 470 | 5-FAM-AhxIWIAQELR#cmlIGD#cmlFNAYYARR-NH2 |
|
| 471 | 5-FAM-AhxRWIAQALR$IGD$FNAFYAHR-NH2 |
|
| 472 | 5-FAM-AhxRWIAQALRRIGDEFNAFYAHR-NH2 |
|
| 473 | 5-FAM-AhxRWIAQALR$IGD$FNAFYARH-NH2 |
|
| 474 | 5-FAM-AhxRWIAQALRRIGDEFNAFYARH-NH2 |
|
| 475 | Ac-RWIAQALR$IGD$FNAFYAAR-NH2 |
|
| 476 | Ac-RWIAQALR$IGD$FNAFYARA-NH2 |
|
| 477 | Ac-RWIAQAAR$DIG$ANAFYARR-NH2 |
|
| 478 | Ac-IWIAQAAR$DIG$ANAFYARR-NH2 |
|
| 479 | 5-FAM-AhxIWIAQELR$IED$FNAYYARR-NH2 |
|
| 480 | 5-FAM-AhxIWIAQELRRIEDEFNAYYARR-NH2 |
|
| 481 | Ac-IWIAQELRNleIGDNleFNAYYARR-NH2 |
|
| 482 | Ac-IWIAQELRAibIGDAibFNAYYARR-NH2 |
|
| 483 | 5-FAM-AhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 484 | 5-FAM-AhxRWIAQALRRIGDEFNAFYARR-NH2 |
|
| 485 | H-CAhxIWIAQALR$IGD$FNAFYARR-NH2 |
|
| 486 | H-CAhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 487 | 5-FAM-AhxIWIAQALR$IGD$FNAFYARR-NH2 |
|
| 488 | OVA-CAhxIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 489 | OVA-CAhxRWIAQQLR$IGD$FNAYYARR-NH2 |
|
| 490 | H-CAhxRWIAQAAR$IGR$ANAFYARR-NH2 |
|
| 491 | H-CAhxRWIAQALR$IGD$FNAYYARR-NH2 |
|
| 492 | H-CAhxIWIAQALRRIGDEFNAYYARR-NH2 |
|
| 493 | OVA-CAhxRWIAQAAR$IGD$ANAYYARR-NH2 |
|
| 494 | OVA-CAhxRWIAQALR$IGD$FNAYYARR-NH2 |
|
| 495 | OVA-CAhxIWIAQALRRIGDEFNAYYARR-NH2 |
|
| 496 | Ac-6xhAhxIWIAQAAR$DIG$ANAYYARR-NH2 |
|
| 497 | Ac-FlagAhxIWIAQAAR$DIG$ANAYYARR-NH2 |
|
| 498 | 5-FAM-6xhAhxIWIAQAAR$DIG$ANAYYARR-NH2 |
|
| 499 | 5-FAM-FlagAhxIWIAQAAR$DIG$ANAYYARR-NH2 |
|
| 500 | Ac-6xhAhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 501 | Ac-FlagAhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 502 | 5-FAM-6xhAhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 503 | 5-FAM-FlagAhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 504 | 5-FAM-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 505 | 5-FAM-BaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 506 | Ac-IWIAQELR%OcoIGD%OcoFNAYYARR-NH2 |
|
| 507 | Ac-AhxIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 508 | Ac-BaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 509 | H-CAhxIWIAQALR$IGD$FNAYYARR-NH2 |
|
| 510 | 5-FAM-AhxIWIAQELR$/IGD$/FNAYYARR-NH2 |
|
| 511 | Ac-RWIAQALRRIGDEFNAFYAHH-NH2 |
|
| 512 | 5-FAM-AhxRWIAQALR$IGD$FNAFYAHH-NH2 |
|
| 513 | 5-FAM-AhxIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 514 | Ac-TatAhxIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 515 | 5-FAM-TatAhxIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 516 | Ac-TatAhxIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 517 | 5-FAM-TatAhxIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 518 | Ac-TatAhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 519 | 5-FAM-TatAhxRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 520 | Ac-TatAhxRWIAQALRRIGDEFNAFYARR-NH2 |
|
| 521 | 5-FAM-TatAhxRWIAQALRRIGDEFNAFYARR-NH2 |
|
| 522 | 5-FAM-AhxRWIAQALR$/IGD$/FNAFYARR-NH2 |
|
| 523 | 5-FAM-AhxIWIAQALR$/IGD$/FNAFYARR-NH2 |
|
| 524 | Ac-TatAhxIWIAQELR$IED$FNAYYARR-NH2 |
|
| 525 | 5-FAM-TatAhxIWIAQELR$IED$FNAYYARR-NH2 |
|
| 526 | Ac-IWIAQELRRIEDDFNAYYARR-NH2 |
|
| 527 | Ac-TatAhxIWIAQELRRIEDDFNAYYARR-NH2 |
|
| 528 | 5-FAM-TatAhxIWIAQELRRIEDDFNAYYARR-NH2 |
|
| 529 | Ac-IWIAQELR$/IED$/FNAYYARR-NH2 |
|
| 530 | 5-FAM-AhxIWIAQELR$/IED$/FNAYYARR-NH2 |
|
| 531 | 5-FAM-AhxIWIAQAAR$DIG$ANAYYARR-NH2 |
|
| 532 | Ac-TatAhxIWIAQAAR$DIG$ANAYYARR-NH2 |
|
| 533 | 5-FAM-TatAhxIWIAQAAR$DIG$ANAYYARR-NH2 |
|
| 534 | Ac-IWIAQAARRDIGEANAYYARR-NH2 |
|
| 535 | Ac-TatAhxIWIAQAARRDIGEANAYYARR-NH2 |
|
| 536 | 5-FAM-TatAhxIWIAQAARRDIGEANAYYARR-NH2 |
|
| 537 | Ac-IWIAQAAR$DIG$ANAYYARR-NH2 |
|
| 538 | 5-FAM-AhxIWIAQAAR$/DIG$/ANAYYARR-NH2 |
|
| 539 | Ac-IWIAQELRRIEDEFNAYYARR-NH2 |
|
| 540 | Ac-IWIAQALR$/IGD$/FNAFYARR-NH2 |
|
| 541 | Ac-RWIAQALR$IGD$FNAFYAHH-NH2 |
|
| 542 | TatAhxIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 543 | 5-FAM-TatAhxIWIAQELRRIEDEFNAYYARR-NH2 |
|
| 544 | Ac-IWIAQALRRI$DEF$AYYARR-NH2 |
|
| 545 | Ac-IWIAQALR$r8IGDEFN$YYARR-NH2 |
|
| 546 | Ac-IWIAQELRRIEDEFNAYYARR-NH2 |
|
| 547 | Ac-IWIAQELR$/IED$/FNAYYARR-NH2 |
|
| 548 | Ac-IWIAQAARRDIGEANAYYARR-NH2 |
|
| 549 | Ac-IWIAQAAR$/DIG$/ANAYYARR-NH2 |
|
| 550 | Ac-IWIAQALR$/IGD$/FNAFYARR-NH2 |
|
| 551 | Ac-RWIAQALR$IGD$FNAFYAHH-NH2 |
|
| 552 | Ac-IWIAQALRRIGDEFNAFYARR-NH2 |
|
| 553 | 5-FAM-AhxIWIAQALR$r8IGDEFN$YYARR-NH2 |
|
| 554 | Ac-RWIAQALR$IGD$FNA-OH |
|
| 555 | Ac-RWIAQALR$IGD$FNAFYA-OH |
|
| 556 | Ac-RWIAQALR$IGD$FNAF-OH |
|
| 557 | Ac-RWIAQALR$IGD$FNAFYARAmr-NH2 |
|
| 558 | 5-FAM-AhxIWIAQALR$/r8IGDEFN$/YYARR-NH2 |
|
| 559 | Ac-IWIAQALR$/r8IGDEFN$/YYARR-NH2 |
|
| 560 | OVA-CAhxIWIAQALR$IGD$FNAYYARR-NH2 |
|
| 561 | Ac-IWIA$ALR$IGDEFNAYYARR-NH2 |
|
| 562 | Ac-IWIA$/ALR$/IGDEFNAYYARR-NH2 |
|
| 563 | 5-FAM-AhxIWIA$/r5ALRSt//IGD$/FNAYYARR-NH2 |
|
| 564 | 5-FAM-AhxIWIA$ALRStIGDEFN$s8YYARR-NH2 |
|
| 565 | HepIAQ$LR$IGD$FNAYYARRTag5-FAM |
|
| 566 | 5-FAM-AhxIWIA$/ALRSt//IGDEFN$/s8YYARR-NH2 |
|
| 567 | 5-FAM-AhxIWIA$r5ALRStIGD$FNAYYARR-NH2 |
|
| 568 | Ac-AAARAAARAAA$AAA$AAAAA-NH2 |
|
| 569 | Ac-AAAAAAAR$AAA$AAAAAARA-NH2 |
|
| 570 | Ac-AAARAAARAAAKAAAEAAAAA-NH2 |
|
| 571 | Ac-AAAAAAARKAAAEAAAAAARA-NH2 |
|
| 572 | Ac-AAARAAAAAARAAAAA-NH2 |
|
| 573 | Ac-IWIAQELR%OIGD%OFNAYYARR-NH2 |
|
| 574 | Ac-IWIA$/r5ALRSt//IGD$/FNAYYARR-NH2 |
|
| 575 | Ac-IWIA$/ALRSt//IGDEFN$/s8YYARR-NH2 |
|
| 576 | Ac-I$r8IAQALR$IGDEFNAYYARR-NH2 |
|
| 577 | Ac-IWIAQALRRIG$r8EFNAYY$RR-NH2 |
|
| 578 | Ac-I$/r8IAQALR$/IGDEFNAYYARR-NH2 |
|
| 579 | Ac-IWIAQALRRIG$/r8EFNAYY$/RR-NH2 |
|
| 580 | Ac-RWIAQALR$IGD$FNAFYAibRR-NH2 |
|
| 581 | Ac-RWIAQALR$IGD$FNASYARR-NH2 |
|
| 582 | Ac-RWIAQALR$r5IGD$r5FNAFYARR-NH2 |
|
| 583 | Ac-IWIAQALRRIGDEF$AYY$RR-NH2 |
|
| 584 | Ac-RWIAEALR$IGD$FNAFYARR-NH2 |
|
| 585 | Ac-RWIAEALR$IGD$FDAFYARR-NH2 |
|
| 586 | Ac-RWIAQALR$/r5IGD$/FNAFYARR-NH2 |
|
| 587 | Ac-RWIAQALR$/IGD$/r5FNAFYARR-NH2 |
|
| 588 | Ac-IWIAQALRRIG$EFN$YYARR-NH2 |
|
| 589 | Ac-IWIAQALRRIGD$FNA$YARR-NH2 |
|
| 590 | Ac-IWIAQALRRIGDE$NAY$ARR-NH2 |
|
| 591 | Ac-IWIAQALRRIGD$r8FNAYYA$R-NH2 |
|
| 592 | %HepIAQ%LR%IGD%FNAYYARR-NH2 |
|
| 593 | Ac-SYDDALLMLRSIGDSL-NH2 |
|
| 594 | Ac-TEMMLAIMLRGIGDSL-NH2 |
|
| 595 | Ac-WVSEFLAIGDYVDFHY-NH2 |
|
| 596 | Ac-DLPVFILRNIGDSLIG-NH2 |
|
| 597 | Ac-VSDFDDFLTSVLDIYL-NH2 |
|
| 598 | 5-FAM-AhxIWIA$ALR$IGDEFNAYYARR-NH2 |
|
| 599 | 5-FAM-AhxIWIAQALRRIGDEF$AYY$RR-NH2 |
|
| 600 | 5-FAM-AhxI$IAQ$LRRIGDEFNAYYARR-NH2 |
|
| 601 | 5-FAM-AhxI$IAQ$LR$IGD$FNAYYARR-NH2 |
|
| 602 | 5-FAM-AhxIWIAQALRRIG$EFN$YYARR-NH2 |
|
| 603 | 5-FAM-AhxIWIAQALRRIGD$FNA$YARR-NH2 |
|
| 604 | 5-FAM-AhxIWIAQALRRIGDE$NAY$ARR-NH2 |
|
| 605 | 5-FAM-AhxI$r8IAQALR$IGDEFNAYYARR-NH2 |
|
| 606 | 5-FAM-AhxIWIAQALRRIGD$r8FNAYYA$R-NH2 |
|
| 607 | 5-FAM-AhxIWIAQALRRIGD$r8FNAYYA$R-NH2 |
|
| 608 | Ac-RWIAQALR$IGD$FDAFYARR-NH2 |
|
| 609 | Ac-IWIA$ALRStIGD$r5FNAYYARR-NH2 |
|
| 610 | Ac-IWIAQALR$IGDStFNA$r5YARR-NH2 |
|
| 611 | Ac-RWIA$ALRStIGD$r5FNAFYARR-NH2 |
|
| 612 | Ac-RWIAQALR$IGDStFNA$r5YARR-NH2 |
|
| 613 | Ac-TENleNleLAINleLR$IGD$L-NH2 |
|
| 614 | Ac-WVSEFL$IGD$VDFHY-NH2 |
|
| 615 | Ac-DLPVFILR$IGD$LIG-NH2 |
|
| 616 | Ac-VSDFDDFLT$VLD$YL-NH2 |
|
| 617 | Ac-RWIAQALR$trIGD$trFNAFYARR-NH2 |
|
| 618 | Ac-RWIAQALR$r5IGDStFNA$YARR-NH2 |
|
| 619 | Ac-RWIAQALR$IGD$FNAibFYARR-NH2 |
|
| 620 | Ac-RWIAQALR$IGD$FNAibFYAibRR-NH2 |
|
| 621 | Ac-PEG3RWIAQALR$IGD$FNAFYARR-NH2 |
|
| 622 | Ac-RWIAQALR$IGD$FNAFYAibHH-NH2 |
|
| 623 | Ac-RWIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 624 | Ac-RWIAQALR$IGD$FNAibFYAibHH-NH2 |
|
| 625 | Ac-RWIAQALR$IGD$FNAAmfYAHH-NH2 |
|
| 626 | Ac-RWIAQALR$r5IGD$FNAFYARR-NH2 |
|
| 627 | Ac-RWIAQALR$IGD$r5FNAFYARR-NH2 |
|
| 628 | Ac-RWIAQALR$IGD$FNAFYARRPEG3-NH2 |
|
| 629 | Ac-RWIAQ$r8LRRIGDStFNAFYA$s8R-NH2 |
|
| 630 | Ac-R$r8IAQALRStIGDEFN$s8FYARR-NH2 |
|
| 631 | Ac-RWIAQALR$IGD$FNADamfYARR-NH2 |
|
| 632 | Ac-RWIAQALRbDIGDbKFNAFYARR-NH2 |
|
| 633 | Ac-RWIAQALRbKIGDbDFNAFYARR-NH2 |
|
| 634 | Ac-RWIAQALR$IAibD$FNAFYARR-NH2 |
|
| 635 | Ac-R$r5IGDStFNA$YARR-NH2 |
|
| 636 | Ac-RWIA$ALRStIGD$r5FNAAmfYARR-NH2 |
|
| 637 | Ac-RWIA$r5ALRStIGD$FNAAmfYARR-NH2 |
|
| 638 | Ac-IWIA$ALRStIGD$r5FNAAmfYARR-NH2 |
|
| 639 | Ac-IWIA$r5ALRStIGD$FNAAmfYARR-NH2 |
|
| 640 | Ac-RWIAQQLR$IGD$FNAFYAHH-NH2 |
|
| 641 | Ac-RWIAQALR#c4IGD#c4FNAFYARR-NH2 |
|
| 642 | Ac-RWIAQALR#c4eIGD#c4eFNAFYARR-NH2 |
|
| 643 | Ac-RWIAQLLR$IGD$FNAFYARR-NH2 |
|
| 644 | Ac-RWIAQALR$IGD$FNAhFYARR-NH2 |
|
| 645 | Ac-RWIAQALR$IGD$FNAAmfYAAmrR-NH2 |
|
| 646 | Biotin-IWIAQELR$IGD$FNAYYARR-NH2 |
|
| 647 | 5-FAM-AhxIWIA$/ALR$/IGDEFNAYYARR-NH2 |
|
| 648 | 5-FAM-AhxRWIAQALR$DIG$FNAFYARR-NH2 |
|
| 649 | Ac-RWIAQALR$IGD$FNAFYARR-OH |
|
| 650 | Ac-IWIAQALR$5a5IGD$5n3FNAYYARR-NH2 |
|
| 651 | Ac-RWIAQQFR$IGD$FNAYYARR-NH2 |
|
| 652 | Ac-RWIAQQLR$IGD$FNAFYAHR-NH2 |
|
| 653 | Ac-RWIAQQLR$IGD$FNAFYARH-NH2 |
|
| 654 | Ac-RWIAQQLRRIGDEFNAFYAHH-NH2 |
|
| 655 | Pr-WIAQQLR$IGD$FNAFYARR-NH2 |
|
| 656 | Ac-WIAQQLR$IGD$FNAYYAR-NH2 |
|
| 657 | Ac-WIAQQLR$IGD$FNAFYAR-NH2 |
|
| 658 | Ac-IWIAQELD$IGD$FNAYYARR-NH2 |
|
| 659 | Ac-RWIAQALD$IGD$FNAFYARR-NH2 |
|
| 660 | Ac-IWIAQLLR$IGD$FNAFYARR-NH2 |
|
| 661 | Ac-RWIAQQLR$IGD$1NalNAYYARR-NH2 |
|
| 662 | Ac-RWIAQLLR$IGD$1NalNAYYARR-NH2 |
|
| 663 | Ac-RWIAQALR$IGD$1NalNAFYARR-NH2 |
|
| 664 | Ac-RWIAQALR$5n3IGD$5a5FNAFYARR-NH2 |
|
| 665 | Ac-RWIAQALR$5a5IGD$5n3FNAFYARR-NH2 |
|
| 666 | Ac-RWIAQALR$/n3IGD$/a5FNAFYARR-NH2 |
|
| 667 | Ac-RWIAQALR$/a5IGD$/n3FNAFYARR-NH2 |
|
| 668 | Pr-WIAQQLR$IGD$FNASYARR-NH2 |
|
| 669 | Pr-NIAQQLR$IGD$FNASYARR-NH2 |
|
| 670 | Pr-SIAQQLR$IGD$FNASYARR-NH2 |
|
| 671 | Pr-WIAQQLR$IGD$FNASYAR-NH2 |
|
| 672 | Ac-RWIAQNLR$IGD$FNAYYARR-NH2 |
|
| 673 | Ac-RWIAQRLR$IGD$FNAYYARR-NH2 |
|
| 674 | Pr-WIAQ$LRR$GDAFNASYARR-NH2 |
|
| 675 | Ac-RWIAQQLR$IGD$FNAYYAHR-NH2 |
|
| 676 | Ac-RWIAQQLR$IGD$FNAYYARH-NH2 |
|
| 677 | Ac-RWIAQQLR$IGD$FNAYYAHH-NH2 |
|
| 678 | Pr-WIAQQLR$IGD$FNASIARR-NH2 |
|
| 679 | Ac-IWIAQQLR$IED$FNAYYARR-NH2 |
|
| 680 | FITC-BaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 681 | FITC-BaIWIAQELD$IGD$FNAYYARR-NH2 |
|
| 682 | FITC-BaRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 683 | FITC-BaRWIAQALD$IGD$FNAFYARR-NH2 |
|
| 684 | HBS-IWAarAQELRRIGDEFNAYYARR-NH2 |
|
| 685 | FITC-BaBaRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 686 | 5-TAMRA-BaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 687 | 5-TAMRA-BaRWIAQALR$IGD$FNAFYARR-NH2 |
|
| 688 | 5-TAMRA-BaIWIAQELR$IED$FNAYYARR-NH2 |
|
| 689 | Ac-RWIAQQLR$IGD$FNASYARR-NH2 |
|
| 690 | Ac-RWIAQQLR$r5IGDStFNA$YARR-NH2 |
|
| 691 | Ac-RWIAQALR$IGD$FNAC13FYARR-NH2 |
|
| 692 | Ac-WIAQQLR$r5IGDStFNA$YARR-NH2 |
|
| 693 | Ac-RIAQELR$IGD$FNAYYAR-NH2 |
|
| 694 | Ac-RIAQQLR$IGD$FNAYYAR-NH2 |
|
| 695 | Ac-RWIA4QAL7R$IGD$FNAFYARR-NH2 |
|
| 696 | Ac-IWIAQELR#cIGR#cFNAYYARR-NH2 |
|
| 697 | Ac-IWIAQELR#cIGD#cFNAYYARR-NH2 |
|
| 698 | Ac-IWIAQELR#5n3IGD#5a5FNAYYARR-NH2 |
|
| 699 | FITC-AhxIWIAQELR#5n3IGD#5a5FNAYYARR-NH2 |
|
| 700 | HepIAQ$LR$IGD$FNAFYARR-NH2 |
|
| 701 | IAQDprLRRIGDEFNAYYARR-NH2 |
|
| 702 | IAQDprLRRIGDQFNAYYARR-NH2 |
|
| 703 | WIADprALRRIGDEFNAYYARR-NH2 |
|
| 704 | WIADprALRRIGDQFNAYYARR-NH2 |
|
| 705 | HepIAQ$LR$IGD$FNAYYARRTag5-FAM- |
|
| 706 | Ac-TENleNleLAINleLR$IGD$L-NH2 |
|
| 707 | 5-TAMRA-BaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 708 | Ac-RWIAQALR$IGD$FNAFYARR-NH2 |
|
| 709 | Ac-IWIAQELR#sIGD#sFNAYYARR-NH2 |
|
| 710 | Ac-IWIAQELR#sIAibD#sFNAYYARR-NH2 |
|
| 711 | Ac-IWIAQELR$sIGD$sFNAYYARR-NH2 |
|
| 712 | HepIAQ$LR$IGD$FNAYYARR-NH2 |
|
| 713 | Ac-RWIAQALR$IGD$VNAFYARR-NH2 |
|
| 714 | Pr-WIAQQLR$IGD$VNAFYARR-NH2 |
|
| 715 | Ac-RWIAQALR$IGD$VNASYARR-NH2 |
|
| 716 | Ac-RWIAQQLR$IGD$VNAFYARR-NH2 |
|
| 717 | Ac-RWIAQQLR$IGD$VNASYARR-NH2 |
|
| 718 | Ac-RWIAQALR$IGD$LNAFYARR-NH2 |
|
| 719 | Ac-RWIAQQLR$IGD$LNAFYARR-NH2 |
|
| 720 | Ac-KALETLRRVGDGV$RNH$TA-NH2 |
|
| 721 | Pr-WIAQQLR$IGD$VNAFYARR-NH2 |
|
| 722 | Pr-WIAQQLR$IGD$VNASYARR-NH2 |
|
| 723 | Ac-RWIAQQLR$IGD$VNAFYAHH-NH2 |
|
| 724 | Pr-WIAQQLR$IGD$VNAFYAR-NH2 |
|
| 725 | Pr-WIAQQLR$IGD$FNAFYAHH-NH2 |
|
| 726 | Pr-WIAQQLR$IGD$FNAFYARH-NH2 |
|
| 727 | Pr-WIAQQLR$IGD$FNAFYAHR-NH2 |
|
| 728 | Ac-RWIA4QAL7R$IGD$FNAFYARR-NH2 |
|
| 729 | Pr-WIAQQLR$IGD$LNAYYARR-NH2 |
|
| 730 | Pr-WIAQQLR$IGD$LNASYARR-NH2 |
|
| 731 | Pr-WIAQQLR$IGD$LNAYYARH-NH2 |
|
| 732 | Pr-WIAQQLR$IGD$LNAYYAHR-NH2 |
|
| 733 | Pr-RIAQQLR$IGD$LNAYYARH-NH2 |
|
| 734 | Pr-RIAQQLR$IGD$LNAYYAHR-NH2 |
|
| 735 | Pr-RIAQQLR$IGD$LNAYYAHH-NH2 |
|
| 736 | Pr-SIAQQLR$IGD$LNAYYARR-NH2 |
|
| 737 | Pr-AibIAQQLR$IGD$LNAYYARR-NH2 |
|
| 738 | Pr-YIAQQLR$IGD$LNAYYARR-NH2 |
|
| 739 | Pr-RIAQQLR$IGD$LNAYYAR-NH2 |
|
| 740 | Ac-RSIAQQLR$IGD$LNAYYARR-NH2 |
|
| 741 | Ac-IWIAQELR$r5IGDStFNA$YARR-NH2 |
|
| 742 | Pr-SIAQQLR$r5IGDStFNA$YARR-NH2 |
|
| 743 | Ac-RWIA$r5ALRStDIL$FNAFYARR-NH2 |
|
| 744 | Ac-RWIAQALR$5a5DIL$5n3FNAFYARR-NH2 |
|
| 745 | Ac-RWIAQQLR$IGD$FNAYYAH-NH2 |
|
| 746 | Ac-RWIA$r5ALRStIDL$FNAFYARR-NH2 |
|
| 747 | Ac-RWIAQALR$5a5ILL$5n3FNAFYARR-NH2 |
|
| 748 | Pr-RIAQQLR$IGD$FNAYYAHH-NH2 |
|
| 749 | Pr-WIAQQLR$IGD$VNAYYAHR-NH2 |
|
| 750 | Pr-WIAQQLR$IGD$VNAFYAHR-NH2 |
|
| 751 | Pr-RIAQQLR$IGD$VNAYYAHR-NH2 |
|
| 752 | Ac-RWIAQALR$5n3DIL$5a5FNAFYARR-NH2 |
|
| 753 | Ac-R$r8IAQALRStIGDLFN$s8FYARR-NH2 |
|
| 754 | Pr-RIAQQLR$IGD$FNAYYAH-NH2 |
|
| 755 | Ac-RWIAQALR$5n3ILL$5a5FNAFYARR-NH2 |
|
| 756 | Ac-RAIAQQLR$IGD$FNAYYAH-NH2 |
|
| 757 | Pr-WIAQQLR$IGD$LNAYYAHH-NH2 |
|
| 758 | Pr-SIAQQLR$IGD$LNAYYAHR-NH2 |
|
| 759 | Ac-RWIAQQLR$IGD$VNAFYAHR-NH2 |
|
| 760 | Ac-IWIA$QLRStIGD$r5FNAYYARR-NH2 |
|
| 761 | Ac-RWIA$QLRStIGD$r5FNAYYARR-NH2 |
|
| 762 | Ac-RWIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 763 | Ac-RWIAQALR$IGD$LNAibFYAHH-NH2 |
|
| 764 | Ac-IWIA$ALRStIGD$r5LNAYYARR-NH2 |
|
| 765 | Ac-IWIAQALR$IGDStFNA$r5YAHH-NH2 |
|
| 766 | Ac-RWIA$ALRStIGD$r5FNAYYARR-NH2 |
|
| 767 | Pr-WIAQQLR$IGD$FNAYYAHH-NH2 |
|
| 768 | Pr-SIAQQLR$IGD$FNAFYARR-NH2 |
|
| 769 | Ac-WIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 770 | Ac-RWIAQALR$IGD$VNAibFYAHH-NH2 |
|
| 771 | Ac-IWIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 772 | Ac-IWIAQALR$IGD$VNAibFYAHH-NH2 |
|
| 773 | Ac-IWIAQALR$IGD$LNAibFYAHH-NH2 |
|
| 774 | Ac-ELR$r5IGDStFNA$YARR-NH2 |
|
| 775 | Ac-QELR$r5IGDStFNA$YARR-NH2 |
|
| 776 | Ac-AQELR$r5IGDStFNA$YARR-NH2 |
|
| 777 | Ac-IAQELR$r5IGDStFNA$YARR-NH2 |
|
| 778 | Ac-RWIAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 779 | Ac-RWIAQQLR$r5IGDStFNA$YAHH-NH2 |
|
| 780 | Ac-RWIAQALR$IGDStFNA$r5YAHH-NH2 |
|
| 781 | Ac-RWIAQQLR$IGDStFNA$r5YAHH-NH2 |
|
| 782 | Ac-IWIAQQFR$IGD$FNAYYARR-NH2 |
|
| 783 | Ac-RWIAQQFR$IGD$FNAFYAHH-NH2 |
|
| 784 | Ac-IWIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 785 | Ac-RWIAQQLR$IGD$FNAibYYAHH-NH2 |
|
| 786 | Ac-IWIAQALR$IGD$FNAibYYAHH-NH2 |
|
| 787 | Ac-RWIAQALR$IGD$FNAibYYAHH-NH2 |
|
| 788 | Ac-RWIAQALR$IGD$LNAibYYAHH-NH2 |
|
| 789 | Ac-RIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 790 | Pr-WIAQQLR$IGD$FNAibYYAHH-NH2 |
|
| 791 | Pr-RIAQQLR$IGD$FNAibYYAHH-NH2 |
|
| 792 | Pr-NIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 793 | Pr-SIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 794 | Pr-NIAQQLR$IGD$FNAibYYARR-NH2 |
|
| 795 | Pr-SIAQQLR$IGD$FNAibYYARR-NH2 |
|
| 796 | Ac-IWIA$r5QLRStIGD$FNAYYARR-NH2 |
|
| 797 | Ac-IWIA$ALDStIGD$r5FNAYYARR-NH2 |
|
| 798 | Ac-RWIAQALD$IGD$FNAibFYAHH-NH2 |
|
| 799 | Ac-RWIAQQLR$IGD$LNAibFYAHH-NH2 |
|
| 800 | Ac-IWIAQQLR$IGD$LNAibFYAHH-NH2 |
|
| 801 | Ac-RAIAQQLR$IGD$LNAibFYAHH-NH2 |
|
| 802 | Ac-IRIAQQLR$IGD$LNAibFYAHH-NH2 |
|
| 803 | Ac-RAIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 804 | Ac-IRIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 805 | Ac-RWIAQALR$IGA$FNAibFYAHH-NH2 |
|
| 806 | Ac-RWIAQQLR$IGA$FNAFYAHH-NH2 |
|
| 807 | Pr-RIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 808 | Pr-WIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 809 | Ac-RWIAQALR$IGD$INAibFYAHH-NH2 |
|
| 810 | Ac-RWIAQALR$IGD$ChgNAibFYAHH-NH2 |
|
| 811 | Ac-IWIAQQLR$IGD$VNAibFYAHH-NH2 |
|
| 812 | Ac-IWIAQQLR$IGD$INAibFYAHH-NH2 |
|
| 813 | Ac-RWIAQQLR$IGD$VNAibFYAHH-NH2 |
|
| 814 | Ac-RWIAQQLR$IGD$INAibFYAHH-NH2 |
|
| 815 | Pr-WIAQQLR$IGD$VNAibFYAHH-NH2 |
|
| 816 | Ac-RWIAQAFR$IGD$VNAibFYAHH-NH2 |
|
| 817 | Ac-RWIAQANleR$IGD$VNAibFYAHH-NH2 |
|
| 818 | Ac-RWIAQAChgR$IGD$VNAibFYAHH-NH2 |
|
| 819 | Ac-RWIAQALR$IGD$LNAFYAibHH-NH2 |
|
| 820 | Ac-RWIAQALR$IGD$VNAFYAibHH-NH2 |
|
| 821 | Ac-RWIAQALD$IGD$FNAibYYAHH-NH2 |
|
| 822 | Ac-RWIA$r5ALRStIGD$FNAYYARR-NH2 |
|
| 823 | Ac-IWIA$r5ALDStIGD$FNAYYARR-NH2 |
|
| 824 | Ac-IWIA$r5ALRStIGD$FNAYYAibRR-NH2 |
|
| 825 | Ac-IWIA$r5ALRStIGD$VNAYYARR-NH2 |
|
| 826 | Ac-IRIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 827 | Ac-INIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 828 | Ac-IFIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 829 | Ac-ISIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 830 | Ac-IAibIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 831 | Ac-IWNleAQALR$IGD$FNAibFYAHH-NH2 |
|
| 832 | Ac-IWIAQANleR$IGD$FNAibFYAHH-NH2 |
|
| 833 | Ac-IWIAibQALR$IGD$FNAibFYAHH-NH2 |
|
| 834 | Pr-IAQALR$IGD$FNAibFYAHH-NH2 |
|
| 835 | Ac-IWIAQAibLR$IGD$FNAibFYAHH-NH2 |
|
| 836 | Ac-IWIAQLLR$IGD$FNAibFYAHH-NH2 |
|
| 837 | Ac-IWIAQFLR$IGD$FNAibFYAHH-NH2 |
|
| 838 | Ac-IAIAAFLR$IGD$FNAibFYA-NH2 |
|
| 839 | Ac-IWIAQALR$IGD$FNAibYYAibHH-NH2 |
|
| 840 | Ac-IWIAQALR$IGD$FAAibFYAHH-NH2 |
|
| 841 | Ac-RWIAQALR$r8IGDAibFN$FYAHH-NH2 |
|
| 842 | Ac-RWIAQALR$r8IGDAFN$FYAHH-NH2 |
|
| 843 | Ac-RWIA$r8ALRAibIG$AFNAibYYAHH-NH2 |
|
| 844 | Ac-RWIA$r8ALRAIG$AFNAibYYAHH-NH2 |
|
| 845 | Ac-IWIAQALR$IGD$ChaNAibFYAHH-NH2 |
|
| 846 | 5-FAM-BaIWIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 847 | 5-FAM-BaRWIAQALR$IGD$LNAibFYAHH-NH2 |
|
| 848 | Ac-IWILQALR$IAibD$FNAibFYAHH-NH2 |
|
| 849 | Ac-IAIAQFLR$IGD$FNAibFYAHH-NH2 |
|
| 850 | Ac-IWIAQALR$r8IGDAFN$FYAHH-NH2 |
|
| 851 | Ac-IWIAQALR$r8IGDAibFN$FYAHH-NH2 |
|
| 852 | Ac-IWIAQNLR$IGD$FNAibFYAHH-NH2 |
|
| 853 | Ac-IWIAQHLR$IGD$FNAibFYAHH-NH2 |
|
| 854 | Ac-RWIAAQLR$IGD$FNAibFYA-NH2 |
|
| 855 | Ac-RNIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 856 | Ac-RFIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 857 | Ac-RAibIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 858 | Ac-RAIAQFLR$IGD$FNAibFYAHH-NH2 |
|
| 859 | Ac-RWIAQLLR$IGD$FNAibFYAHH-NH2 |
|
| 860 | Ac-RWIAQFLR$IGD$FNAibFYAHH-NH2 |
|
| 861 | Ac-RWIAQAibLR$IGD$FNAibFYAHH-NH2 |
|
| 862 | Ac-RWIAQALR$IGD$FNAibFYQHH-NH2 |
|
| 863 | Ac-RWIAQHLR$IGD$FNAibFYAHH-NH2 |
|
| 864 | Ac-RWIAQALR$NleGD$FNAibFYAHH-NH2 |
|
| 865 | Pr-IAQLLR$IGD$FNAibFYAHH-NH2 |
|
| 866 | Ac-RWIALALR$IGD$FNAibFYAHH-NH2 |
|
| 867 | Pr-WIALALR$IGD$FNAibFYAHH-NH2 |
|
| 868 | Ac-RAIAFALR$IGD$FNAibFYAHH-NH2 |
|
| 869 | Ac-WIAQALR$IGD$FNAibFYQHH-NH2 |
|
| 870 | Ac-CCPGCCBaIWIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 871 | Ac-CCPGCCBaRWIAQALR$IGD$VNAibFYAHH-NH2 |
|
| 872 | Ac-CCPGCCBaRWIAQALR$IGD$LNAibFYAHH-NH2 |
|
| 873 | Ac-IWIAQALR$IGD$FNAibFYQHH-NH2 |
|
| 874 | Ac-RWIAQAibLR$r5IGDStFNA$YAHH-NH2 |
|
| 875 | Ac-IWIAQLLR$IGD$FNAibFYQHH-NH2 |
|
| 876 | Ac-RWIAQALR$IGD$FNRFYAHH-NH2 |
|
| 877 | Ac-RWIAQALR$IGD$FNAFYRHH-NH2 |
|
| 878 | Ac-RWIAQRLR$IGD$FNAFYAHH-NH2 |
|
| 879 | Ac-RWIAQALR$IGD$FNARYAHH-NH2 |
|
| 880 | Ac-RWIAERLR$IGD$FNAFYAHH-NH2 |
|
| 881 | Ac-RWIAQALR$IGD$FNQFYAHH-NH2 |
|
| 882 | Ac-RWIAQALR$IGD$FNAFYQHH-NH2 |
|
| 883 | Ac-RWIAQELR$IGD$FNARYAHH-NH2 |
|
| 884 | Ac-RWIAQALR$IGD$FNAQYAHH-NH2 |
|
| 885 | Ac-RWIAQQLR$IGD$QNQQYQHH-NH2 |
|
| 886 | Ac-IWIAAFLR$IGD$FNAibFYAHH-NH2 |
|
| 887 | Ac-IWIAQALR$IGD$FNleAibFYAHH-NH2 |
|
| 888 | Ac-IWIAQALR$IGD$FNleAibFYQHH-NH2 |
|
| 889 | Ac-IWIAQAibLR$IGD$VNAibFYAHH-NH2 |
|
| 890 | Ac-IWIAQLLR$IGD$VNAibFYAHH-NH2 |
|
| 891 | Ac-IWIAQAAR$IGD$VNAibFYAHH-NH2 |
|
| 892 | Ac-IAIAFALR$IGD$VNAibFYAHH-NH2 |
|
| 893 | Ac-IWIALALR$IGD$VNAibFYAHH-NH2 |
|
| 894 | Ac-IWIAQALR$IGD$VNAibFYQHH-NH2 |
|
| 895 | Ac-IWIAQELR$4n4IGD$4a3FNAYYARR-NH2 |
|
| 896 | Ac-IWIAQELR$4a3IGD$4n4FNAYYARR-NH2 |
|
| 897 | Ac-IWIAQELR$4n3IGD$4a5FNAYYARR-NH2 |
|
| 898 | Ac-IWIAQELR$4a5IGD$4n3FNAYYARR-NH2 |
|
| 899 | Ac-IWIAQELR$4n5IGD$4a5FNAYYARR-NH2 |
|
| 900 | Ac-IWIAQELR$4a5IGD$4n5FNAYYARR-NH2 |
|
| 901 | Ac-RCouIAQALR$IGD$LNAibFYAHH-NH2 |
|
| 902 | Ac-RCouIAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 903 | Ac-ICouIAQALRRIGDELNAibFYAHH-NH2 |
|
| 904 | Ac-RCouIAQALRRIGDEFNAFYAHH-NH2 |
|
| 905 | Ac-IWIAQALR$IGD$FNAFYAibHH-NH2 |
|
| 906 | Ac-IWIALALR$IGD$FNAibFYAHH-NH2 |
|
| 907 | Ac-IAIAFALR$IGD$FNAibFYAHH-NH2 |
|
| 908 | Ac-RWIAQHLR$IGD$VNAibFYAHH-NH2 |
|
| 909 | Ac-IWIAQHLR$IGD$VNAibFYAHH-NH2 |
|
| 910 | Ac-RWIAQLLR$IGD$VNAibFYAHH-NH2 |
|
| 911 | Ac-IWIAQLLR$IGD$VNAibFYAHH-NH2 |
|
| 912 | Ac-IWIAQFLR$IGD$VNAibFYAHH-NH2 |
|
| 913 | Ac-IWIAQALR$IGD$HNAibFYAHH-NH2 |
|
| 914 | Ac-IWIAHLLR$IGD$VNAibFYAHH-NH2 |
|
| 915 | Ac-IWIAQALR$IGD$INAibFYAHH-NH2 |
|
| 916 | Ac-IWIAQLLR$IGD$INAibFYAHH-NH2 |
|
| 917 | Ac-IHIAQLLR$IGD$FNAibFYAHH-NH2 |
|
| 918 | Ac-IHIAQLLR$IGD$VNAibFYAHH-NH2 |
|
| 919 | Ac-IWIAQLLR$IGD$VNAibFYAHA-NH2 |
|
| 920 | Ac-IWIAQLLR$IGD$VNAibFYAAH-NH2 |
|
| 921 | Ac-RWIAQALD$IGR$VNAibFYAHH-NH2 |
|
| 922 | Ac-RWIAQALD$IGD$VNAibFYAHH-NH2 |
|
| 923 | Ac-IWIAQALD$IGR$VNAibFYAHH-NH2 |
|
| 924 | Ac-RWIAQAAR$IAibD$VNAibFYAHH-NH2 |
|
| 925 | Ac-IWIAQALD$IGR$FNAibFYAHH-NH2 |
|
| 926 | Ac-IWIAQALD$IGD$FNAibFYAHH-NH2 |
|
| 927 | Ac-IWIAQAAR$IAibD$FNAibFYAHH-NH2 |
|
| 928 | Ac-RWIAQALD$r5IGRStFNA$YAHH-NH2 |
|
| 929 | Ac-IWIAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 930 | Ac-RWIAAQLR$IGD$VNAibFYAHH-NH2 |
|
| 931 | Ac-IWIAAQLR$IGD$FNAibFYAHH-NH2 |
|
| 932 | Ac-IWNleAQLLR$IGD$FNAibFYAHH-NH2 |
|
| 933 | Ac-RWNleAQLLR$IGD$VNAibFYAHH-NH2 |
|
| 934 | Ac-IWNleAibQLLR$IGD$FNAibFYAHH-NH2 |
|
| 935 | Ac-RWNleAibQLLR$IGD$VNAibFYAHH-NH2 |
|
| 936 | Ac-IRIAQLLR$IGD$FNAibFYAHH-NH2 |
|
| 937 | Ac-ISIAQLLR$IGD$FNAibFYAHH-NH2 |
|
| 938 | Ac-IRIAibQLLR$IGD$FNAibFYAHH-NH2 |
|
| 939 | Ac-ISIAibQLLR$IGD$FNAibFYAHH-NH2 |
|
| 940 | Ac-IWIA$r5ALDStIGR$FNAYYARR-NH2 |
|
| 941 | Pr-WIAibQLLR$IGD$FNAibFYAibHH-NH2 |
|
| 942 | Ac-IWIAibQLLR$IGD$VNAibFYAibHH-NH2 |
|
| 943 | Pr-WIAQLLR$IGD$VNAibFYAibHH-NH2 |
|
| 944 | Pr-WIAibQALR$IGD$FNAibFYAibHH-NH2 |
|
| 945 | Ac-IWIAibQALR$IGD$VNAibFYAibHH-NH2 |
|
| 946 | Ac-RWIAibQALR$IGD$VNAibFYAibHH-NH2 |
|
| 947 | Ac-IWIAQAibLR$IGD$FNAibFYAibHH-NH2 |
|
| 948 | Ac-IWIAQAibLR$IGD$VNAibFYAibHH-NH2 |
|
| 949 | Ac-RWIAQAibLR$IGD$VNAibFYAibHH-NH2 |
|
| 950 | Ac-IWIAQALR$IGD$VNAibFYAibHH-NH2 |
|
| 951 | FITC-BaIWIAQELR$IGD$F |
|
| 952 | Ac-I$IAQ$LRRIGDEF$AYY$R-NH2 |
|
| 953 | Ac-I$IAQ$LRNleIGDNleF$AYY$R-NH2 |
|
| 954 | Ac-I$IAQ$LRRIGDEF$AYY$HH-NH2 |
|
| 955 | Ac-I$IAQ$LRNleIGDNleF$AYY$HH-NH2 |
|
| 956 | Ac-IWIA$ALR$IGD$FNA$YARR-NH2 |
|
| 957 | Ac-IWIA$ALR$IGD$FNA$YAHH-NH2 |
|
| 958 | Ac-IWIA$ALR$IGD$FNA$YAR-NH2 |
|
| 959 | Ac-IWIAQ$LRA$GDAFNAYYAR-NH2 |
|
| 960 | Ac-IWIAQ$LRA$GDAFNAYYAHH-NH2 |
|
| 961 | Ac-IWIAQALR$r8IGDAFN$YYARR-NH2 |
|
| 962 | Ac-IWIAQALR$r8IGDNleFN$YYARR-NH2 |
|
| 963 | Ac-IWIAQALR$r8IGDAibFN$YYARR-NH2 |
|
| 964 | Ac-IWIAQALR$r8IGDAFN$YYAHH-NH2 |
|
| 965 | Ac-IWIAQALR$r8IGDNleFN$YYAHH-NH2 |
|
| 966 | Ac-IWIAQALR$r8IGDAibFN$YYAHH-NH2 |
|
| 967 | Ac-IWIAQALR$r8IGDAFN$YYAR-NH2 |
|
| 968 | Ac-ICouIAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 969 | Ac-ICouIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 970 | Ac-ICouIAQELR$IGD$FNAibFYAHH-NH2 |
|
| 971 | Ac-ICouIAQALD$IGR$FNAibFYAHH-NH2 |
|
| 972 | Ac-ICouIAQALR$IGD$FNAibFYAAA-NH2 |
|
| 973 | Ac-ICouIAQALR$IGD$FNAibFYA-NH2 |
|
| 974 | Ac-RCou2IAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 975 | Ac-RCou2IAQQLR$r5IGDStFNA$YAHH-NH2 |
|
| 976 | Ac-RCou2IAQALR$IGD$LNAibFYAHH-NH2 |
|
| 977 | Ac-ICou2IAQALR$IGD$FNAibFYAHH-NH2 |
|
| 978 | Ac-ICou2IAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 979 | Ac-RWIAQALR$5rn3IGDSta5FNA$5n3YAHH-NH2 |
|
| 980 | Ac-RCou3IAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 981 | Ac-RCou3IAQQLR$r5IGDStFNA$YAHH-NH2 |
|
| 982 | Ac-RCou3IAQALR$IGD$LNAibFYAHH-NH2 |
|
| 983 | Ac-ICou3IAQALR$IGD$FNAibFYAHH-NH2 |
|
| 984 | Ac-ICou3IAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 985 | Ac-IWIAQALR$IGD$FNAibFYAAA-NH2 |
|
| 986 | Ac-IWIAQELR$IGD$FNAibFYAHH-NH2 |
|
| 987 | Ac-IWIAQALR$r8IGAAibFN$FYAHH-NH2 |
|
| 988 | Ac-IWIAQALR$IGD$FNAibFYA-NH2 |
|
| 989 | Ac-ICou2IA$ALRStIGD$r5FNAYYARR-NH2 |
|
| 990 | Ac-IDprIA$ALRStIGD$r5FNAYYARR-NH2 |
|
| 991 | Ac-ICou2IA$QLRStIGD$r5FNAYYARR-NH2 |
|
| 992 | Ac-IDprIA$QLRStIGD$r5FNAYYARR-NH2 |
|
| 993 | Ac-IWIAQQLR$r5IGDStFNA$YAHH-NH2 |
|
| 994 | Ac-ICou2IAQQLR$r5IGDStFNA$YAHH-NH2 |
|
| 995 | Ac-IDprIAQQLR$r5IGDStFNA$YAHH-NH2 |
|
| 996 | Ac-RDprIAQQLR$r5IGDStFNA$YAHH-NH2 |
|
| 997 | Ac-IWIAQALR$IGD$FNAibCou2YAHH-NH2 |
|
| 998 | Ac-IWIAQALR$IGD$FNAibCou3YAHH-NH2 |
|
| 999 | Ac-IWIAQALR$IGD$FNAibDprYAHH-NH2 |
|
| 1000 | Ac-IRIAQALR$IGD$FNAibCou2YAHH-NH2 |
|
| 1001 | Ac-IRIAQALR$IGD$FNAibCou3YAHH-NH2 |
|
| 1002 | Ac-IRIAQALR$IGD$FNAibDprYAHH-NH2 |
|
| 1003 | Ac-IAibIAQALR$IGD$FNAibCou2YAHH-NH2 |
|
| 1004 | Ac-IAibIAQALR$IGD$FNAibCou3YAHH-NH2 |
|
| 1005 | Ac-IAibIAQALR$IGD$FNAibDprYAHH-NH2 |
|
| 1006 | Ac-ICou2IAQALR$IGD$FAAibFYAHH-NH2 |
|
| 1007 | Ac-ICou3IAQALR$IGD$FAAibFYAHH-NH2 |
|
| 1008 | Ac-IDprIAQALR$IGD$FAAibFYAHH-NH2 |
|
| 1009 | Pam-IWIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 1010 | Pam-ICou2IAQALR$IGD$FNAibFYAHH-NH2 |
|
| 1011 | Pam-ICou3IAQALR$IGD$FNAibFYAHH-NH2 |
|
| 1012 | Pam-IDprIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 1013 | Ac-IWIAQALR$5n3IGD$5a5FNAibFYAHH-NH2 |
|
| 1014 | Ac-IWIAQALR$5a5IGD$5n3FNAibFYAHH-NH2 |
|
| 1015 | Ac-IWIAQALR$r8IGDAFN$YYARR-NH2 |
|
| 1016 | Ac-ICou2IAQELR$IGD$FNAibFYAHH-NH2 |
|
| 1017 | Ac-ICou2IAQALD$IGR$FNAibFYAHH-NH2 |
|
| 1018 | Ac-ICou2IAQALR$IGD$FNAibFYAAA-NH2 |
|
| 1019 | Ac-ICou2IAQALR$IGD$FNAibFYA-NH2 |
|
| 1020 | Ac-RCou2IAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 1021 | Ac-RCou2IAQALR$IGD$FNAibFYAHH-NH2 |
|
| 1022 | Ac-RCou2IAQELR$IGD$FNAibFYAHH-NH2 |
|
| 1023 | Ac-RCou2IAQALD$IGR$FNAibFYAHH-NH2 |
|
| 1024 | Ac-RCou2IAQALR$IGD$FNAibFYAAA-NH2 |
|
| 1025 | Ac-RCou2IAQALR$IGD$FNAibFYA-NH2 |
|
| 1026 | Ac-IWIAQALR$r8IGAAibFN$FYAHH-NH2 |
|
| 1027 | Ac-IWIA$ALRStIGD$r5FNAYYARR-NH2 |
|
| 1028 | Pr-Cou2IAQALR$IGD$FNAibFYAHH-NH2 |
|
| 1029 | Pr-Cou2IAQALR$IGD$FNAibFYQHH-NH2 |
|
| 1030 | Ac-RWIAQELR$IGD$FNAibFYAHH-NH2 |
|
| 1031 | Ac-RWIAQALD$IGR$FNAibFYAHH-NH2 |
|
| 1032 | Ac-RWIAQALR$IGD$FNAibFYAAA-NH2 |
|
| 1033 | Ac-RWIAQALR$IGD$FNAibFYA-NH2 |
|
| 1034 | Ac-ICou2IAQALRRIGDEFNAYYAHH-NH2 |
|
| 1035 | Ac-ICou2IAQELR$IGD$FNAibFYAHH-NH2 |
|
| 1036 | Ac-ICou2IAQALD$IGR$FNAibFYAHH-NH2 |
|
| 1037 | Ac-ICou4IAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 1038 | Ac-RCou4IAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 1039 | Ac-ICou4IAQALR$IGD$FNAibFYAHH-NH2 |
|
| 1040 | Ac-ICou4IAQQLR$IGD$FNAibFYAHH-NH2 |
|
| 1041 | Ac-RCou4IAQALR$IGD$LNAibFYAHH-NH2 |
|
| 1042 | Ac-IWIAQALR$5a5IGD$5n3FNAibFYAHH-NH2 |
|
| 1043 | Ac-RWIAQALR$/rn3IGDSta/FNA$/n3YAHH-NH2 |
|
| 1044 | Ac-ICou2IA$r5ALRStIGD$FNAYYARR-NH2 |
|
| 1045 | Ac-ICou2IA$r5QLRStIGD$FNAYYARR-NH2 |
|
| 1046 | Ac-ICou4IA$r5ALRStIGD$FNAYYARR-NH2 |
|
| 1047 | Ac-ICou4IA$r5QLRStIGD$FNAYYARR-NH2 |
|
| 1048 | Ac-RCou2IAQALR$IGDStFNA$r5YAHH-NH2 |
|
| 1049 | Ac-RCou4IAQALR$IGDStFNA$r5YAHH-NH2 |
|
| 1050 | Ac-ICou7IAQQLR$r5IGDStFNA$YAHH-NH2 |
|
| 1051 | Ac-RCou7IAQQLR$r5IGDStFNA$YAHH-NH2 |
|
| 1052 | Ac-IWIAQALR$IGD$FNAibCou7YAHH-NH2 |
|
| 1053 | Ac-IRIAQALR$IGD$FNAibCou7YAHH-NH2 |
|
| 1054 | Ac-ICou2IAQQLR$r5IGDStFNA$YAHH-NH2 |
|
| 1055 | Ac-AAIAQALR$IGD$FNAibFYAHH-NH2 |
|
| 1056 | Ac-AAIAQALR$IGD$FNAibFYA-NH2 |
|
| 1057 | Ac-IWIAQALR$IGD$FNAibFYAAAAa-NH2 |
|
| 1058 | Ac-IWIAQALR$IGD$FNAibAAAAAa-NH2 |
|
| 1059 | Ac-IWIAQALR$IGD$FNAibFYAHHAAAAa-NH2 |
|
| 1060 | Ac-IWIAQALA$IGD$FNAibFYAHH-NH2 |
|
| 1061 | Ac-IWIAQALR$IGD$FAAibFYA-NH2 |
|
| 1062 | Ac-IWIALALR$IGD$FAAibFYA-NH2 |
|
| 1063 | Ac-IWIALALR$IGD$FNAibFYA-NH2 |
|
| 1064 | Ac-IWIALALR$IGD$FAAibFYAHH-NH2 |
|
| 1065 | Ac-IWIALALR$IGD$FAAAAA-NH2 |
|
| 1066 | Ac-IWIALALR$IGD$FNAAAA-NH2 |
|
| 1067 | Ac-IWIALLLR$IGD$FAAibFYAHH-NH2 |
|
| 1068 | Ac-IWIALLLR$IGD$FNAibFYAHH-NH2 |
|
| 1069 | Ac-IWIALLLR$IGD$FNAibFYA-NH2 |
|
| 1070 | Ac-IWIALLLR$IGD$FNAibFYAAAAAa-NH2 |
|
| 1071 | Ac-RWIALQLR$r5IGDStFNA$YAHH-NH2 |
|
| 1072 | Ac-RWIAQQLR$r5IGDStFNA$YA-NH2 |
|
| 1073 | Ac-RWIAQQLR$r5IGDStFNA$YAAa-NH2 |
|
| 1074 | Ac-RWIALQLR$r5IGDStFNA$YAAa-NH2 |
|
| 1075 | Ac-RCou2IALQLR$r5IGDStFNA$YAHH-NH2 |
|
| 1076 | Ac-RCou2IAQQLR$r5IGDStFNA$YA-NH2 |
|
| 1077 | Ac-RCou2IAQQLR$r5IGDStFNA$YAAa-NH2 |
|
| 1078 | Ac-RCou2IALQLR$r5IGDStFNA$YAAa-NH2 |
|
| 1079 | Ac-RCou2IAQALR$5rn3IGDSta5FNA$5n3YAHH-NH2 |
|
| 1080 | RCou4IAQALR$5rn3IGDSta5FNA$5n3YAHH-NH2 |
|
| 1081 | 5-FAM-BaRWIAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 1082 | Ac-RCou2IAQQLRAibIGDAibFNAAibYAHH-NH2 |
|
| 1083 | Ac-RWIAQQLRAibIGDAibFNAAibYAHH-NH2 |
|
| 1084 | Ac-RCou2IAQELR$r5IGDStFNA$YAHH-NH2 |
|
| 1085 | Ac-RWIAQELR$r5IGDStFNA$YAHH-NH2 |
|
| 1086 | Ac-ICou2IAQELR$IGD$FNAYYARR-NH2 |
|
| 1087 | Ac-IWIAQALR4Me$5a5IGD$5n3FNAibFYAHH-NH2 |
|
| 1088 | Ac-IWIAQALR4Ph$5a5IGD$5n3FNAibFYAHH-NH2 |
|
| 1089 | Ac-NleWIAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 1090 | Ac-KWIAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 1091 | Ac-RWIAQALR$r5IGDStFNA$YQHH-NH2 |
|
| 1092 | Ac-IWIAQALR$r5IGDStFNA$YQHH-NH2 |
|
| 1093 | Ac-NleCou2IAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 1094 | Ac-KCou2IAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 1095 | Ac-IWIAQELRRIGDEF$AYY$RR-NH2 |
|
| 1096 | Ac-IWIAQELRRIGDEFN$YYA$R-NH2 |
|
| 1097 | Ac-IWIAQEL$r8RIGDEF$AYYARR-NH2 |
|
| 1098 | Ac-IWIAQELR$r8IGDEFN$YYARR-NH2 |
|
| 1099 | Ac-IWIAQELRRIGD$r8FNAYYA$R-NH2 |
|
| 1100 | Ac-I$IAQStLRRIGD$s8FNAYYARR-NH2 |
|
| 1101 | Ac-I$r8IAQELRStIGD$r5FNAYYARR-NH2 |
|
| 1102 | Ac-I$r8IAQELRStIGDEFN$s8YYARR-NH2 |
|
| 1103 | Ac-IWI$QELStRIGDEF$s8AYYARR-NH2 |
|
| 1104 | Ac-IWIA$ELRStIGD$r5FNAYYARR-NH2 |
|
| 1105 | Ac-IWIA$r5ELRStIGD$FNAYYARR-NH2 |
|
| 1106 | Ac-IWIA$ELRStIGDEFN$s8YYARR-NH2 |
|
| 1107 | Ac-IWIAQ$r8LRRIGDStFNAYYA$s8R-NH2 |
|
| 1108 | Ac-IWIAQEL$r8RIGDEFStAYY$r5RR-NH2 |
|
| 1109 | Ac-IWIAQELR$IGDStFNAYYA$s8R-NH2 |
|
| 1110 | Ac-IWIAQELR$r8IGDEFNStYYA$r5R-NH2 |
|
| 1111 | Ac-I$IAQ$LRRIGDEF$AYY$RR-NH2 |
|
| 1112 | Ac-I$IAQ$LRRIGDEFN$YYA$R-NH2 |
|
| 1113 | Ac-IWI$QEL$RIGDEF$AYY$RR-NH2 |
|
| 1114 | Ac-IWI$QEL$RIGDEFN$YYA$R-NH2 |
|
| 1115 | Ac-IWIA$ELR$IGDEF$AYY$RR-NH2 |
|
| 1116 | Ac-IWIA$ELR$IGDEFN$YYA$R-NH2 |
|
| 1117 | Ac-I$r8IAQELR$IGDEF$AYY$RR-NH2 |
|
| 1118 | Ac-I$r8IAQELR$IGDEFN$YYA$R-NH2 |
|
| 1119 | Ac-IWIAQ$r8LRRIGD$F$AYY$RR-NH2 |
|
| 1120 | Ac-IWIAQ$r8LRRIGD$FN$YYA$R-NH2 |
|
| 1121 | Ac-I$IAQ$L$r8RIGDEF$AYYARR-NH2 |
|
| 1122 | Ac-I$IAQ$LR$r8IGDEFN$YYARR-NH2 |
|
| 1123 | Ac-I$IAQ$LRRIGD$r8FNAYYA$R-NH2 |
|
| 1124 | Ac-IWI$QEL$RIGD$r8FNAYYA$R-NH2 |
|
| 1125 | Ac-IWIA$ELR$IGD$r8FNAYYA$R-NH2 |
|
| 1126 | 5-FAM-BaIWIAQELRRIGDEFNAYYARR-NH2 |
|
| 1127 | 5-FAM-BaIWIAQELR$IGD$FNAYYARR-NH2 |
|
| 1128 | 5-FAM-BaNLWAAQRYGRELR$NleSD$FVDSFKK-NH2 |
|
| 1129 | 5-FAM-BaKALETLR$VGD$VQRNHETAF-NH2 |
|
| 1130 | Ac-RCou2IAQALR$IGD$FNAFYARR-NH2 |
|
| 1131 | Ac-RCou2IAQALR$5rn3IGDSta5FNA$5n3YAHH-NH2 |
|
| 1132 | Ac-IWI$QEL$RIGDEF$AYY$RR-NH2 |
|
| 1133 | Ac-IWIAQ$r8LRRIGD$F$AYY$RR-NH2 |
|
| 1134 | Ac-IWIAQ$r8LRRIGD$FN$YYA$R-NH2 |
|
| 1135 | Ac-IWI$QEL$RIGD$r8FNAYYA$R-NH2 |
|
| 1136 | Ac-IWIA$ELR$IGD$r8FNAYYA$R-NH2 |
|
| 1137 | Ac-IWI$QELStRIGDEF$s8AYYARR-NH2 |
|
| 1138 | Ac-IWIAQ$r8LRRIGDStFNAYYA$s8R-NH2 |
|
| 1139 | Ac-IWIAQEL$r8RIGDEFStAYY$r5RR-NH2 |
|
| 1140 | Ac-I$r8IAQELR$IGDEF$AYY$RR-NH2 |
|
| 1141 | Ac-IWIAQ$r8LRRIGD$FNAYYARR-NH2 |
|
| 1142 | Ac-IWIAQELRRIGDEF$AYY$RR-NH2 |
|
| 1143 | Ac-IWIAQALR$r8IGDAFN$YYA-NH2 |
|
| 1144 | Ac-WIAQALR$r8IGDAFN$YYA-NH2 |
|
| 1145 | Ac-IAQALR$r8IGDAFN$YYA-NH2 |
|
| 1146 | Ac-IAAALR$r8IGDAFN$YYA-NH2 |
|
| 1147 | Ac-IAQALA$r8IGDAFN$YYA-NH2 |
|
| 1148 | Ac-IAQALR$r8IADAFN$YYA-NH2 |
|
| 1149 | Ac-IAQALR$r8IGDAAN$YYA-NH2 |
|
| 1150 | Ac-IAQALR$r8IGDAFA$YYA-NH2 |
|
| 1151 | Ac-IAQALR$r8IGDAFN$AYA-NH2 |
|
| 1152 | Ac-IAQALR$r8IGDAFN$YAA-NH2 |
|
| 1153 | Ac-IAQALRRIGDEFNAYYAHH-NH2 |
|
| 1154 | Ac-IAQALR$IGD$FNAYYAHH-NH2 |
|
| 1155 | Ac-IWIAQALRRIGDEFNAYYAHH-NH2 |
|
| 1156 | Ac-IWIAQALR$IGD$FNAYYAHH-NH2 |
|
| 1157 | Ac-I$IAQ$LR$IGD$FNAYYAHH-NH2 |
|
| 1158 | HepIAQ$LRRIGDEFNAYYAHH-NH2 |
|
| 1159 | HepIAQ$LR$IGD$FNAYYAHH-NH2 |
|
| 1160 | HepIA$ALRRIGDEFNAYYAHH-NH2 |
|
| 1161 | HepIA$ALR$IGD$FNAYYAHH-NH2 |
|
| 1162 | Ac-I$IAQ$LRRIGDEF$AYY$AA-NH2 |
|
| 1163 | Ac-I$IAQ$LRRIGDEF$AYY$A-NH2 |
|
| 1164 | Ac-I$IAA$LRRIGDEF$AYY$A-NH2 |
|
| 1165 | Ac-I$IAV$LRRIGDEF$AYY$A-NH2 |
|
| 1166 | Ac-I$IAL$LRRIGDEF$AYY$A-NH2 |
|
| 1167 | Ac-I$IAI$LRRIGDEF$AYY$A-NH2 |
|
| 1168 | Ac-I$IAF$LRRIGDEF$AYY$A-NH2 |
|
| 1169 | Ac-I$IAY$LRRIGDEF$AYY$A-NH2 |
|
| 1170 | Ac-I$IAG$LRRIGDEF$AYY$A-NH2 |
|
| 1171 | Ac-I$IAQ$LRAIGDAF$AYY$A-NH2 |
|
| 1172 | Ac-I$IAQ$LRAIGDAibF$AYY$A-NH2 |
|
| 1173 | Ac-I$IAQ$LRAibIGDAF$AYY$A-NH2 |
|
| 1174 | Ac-I$IAQ$LRAibIGDAibF$AYY$A-NH2 |
|
| 1175 | Ac-I$IAQ$LRNleIGDNleF$AYY$A-NH2 |
|
| 1176 | Ac-I$IAQ$LRNleIGDAibF$AYY$A-NH2 |
|
| 1177 | Ac-I$IAQ$LRAibIGDNleF$AYY$A-NH2 |
|
| 1178 | Ac-I$IAQ$LR$r8IGDEFN$YYA-NH2 |
|
| 1179 | Ac-I$IAA$LR$r8IGDEFN$YYA-NH2 |
|
| 1180 | Ac-I$IAV$LR$r8IGDEFN$YYA-NH2 |
|
| 1181 | Ac-I$IAL$LR$r8IGDEFN$YYA-NH2 |
|
| 1182 | Ac-I$IAI$LR$r8IGDEFN$YYA-NH2 |
|
| 1183 | Ac-I$IAF$LR$r8IGDEFN$YYA-NH2 |
|
| 1184 | Ac-I$IAY$LR$r8IGDEFN$YYA-NH2 |
|
| 1185 | Ac-I$IAG$LR$r8IGDEFN$YYA-NH2 |
|
| 1186 | Ac-I$IAQ$LR$r8IGDAFN$YYA-NH2 |
|
| 1187 | Ac-I$IAQ$LR$r8IGDNleFN$YYA-NH2 |
|
| 1188 | Ac-I$IAQ$LR$r8IGDAibFN$YYA-NH2 |
|
| 1189 | Ac-IWIA$ELR$IGD$r8FNAYYA$A-NH2 |
|
| 1190 | Ac-IWIA$ALR$IGD$r8FNAYYA$A-NH2 |
|
| 1191 | Ac-IWIA$VLR$IGD$r8FNAYYA$A-NH2 |
|
| 1192 | Ac-IWIA$LLR$IGD$r8FNAYYA$A-NH2 |
|
| 1193 | Ac-IWIA$ILR$IGD$r8FNAYYA$A-NH2 |
|
| 1194 | Ac-IWIA$FLR$IGD$r8FNAYYA$A-NH2 |
|
| 1195 | Ac-IWIA$YLR$IGD$r8FNAYYA$A-NH2 |
|
| 1196 | Ac-IWIA$GLR$IGD$r8FNAYYA$A-NH2 |
|
| 1197 | Ac-IWIA$SLR$IGD$r8FNAYYA$A-NH2 |
|
| 1198 | Ac-I$IAQ$LRRIGDEF$AYY$-NH2 |
|
| 1199 | Ac-IWIA$ELR$IGD$r8FNAYYA$-NH2 |
|
| 1200 | Ac-WIAQALR$r8IGDAFN$YYA-NH2 |
|
| 1201 | Ac-IAQALR$r8IGDAFN$YYA-NH2 |
|
| 1202 | Ac-IAAALR$r8IGDAFN$YYA-NH2 |
|
| 1203 | Ac-IAQALA$r8IGDAFN$YYA-NH2 |
|
| 1204 | Ac-IAQALR$r8IADAFN$YYA-NH2 |
|
| 1205 | Ac-IAQALR$r8IGDAAN$YYA-NH2 |
|
| 1206 | Ac-IAQALR$r8IGDAFA$YYA-NH2 |
|
| 1207 | Ac-IAQALR$r8IGDAFN$AYA-NH2 |
|
| 1208 | Ac-IAQALR$r8IGDAFN$YAA-NH2 |
|
| 1209 | Ac-I$IAL$LR$r8IGDAFN$YYA-NH2 |
|
| 1210 | Ac-I$IALALR$IGDAFN$YYA$A-NH2 |
|
| 1211 | Ac-IWIA$ALR$IGDAFN$YYA$A-NH2 |
|
| 1212 | Ac-IWIA$ALRStIGDAFN$s8YYA-NH2 |
|
| 1213 | Ac-IWIA$ALRStIGDNleFN$s8YYA-NH2 |
|
| 1214 | Ac-I$r8IALALRStIGDAFN$s8YYA-NH2 |
|
| 1215 | Ac-I$r8IALALRStIGD$r5FNAYYA-NH2 |
|
| 1216 | Ac-IWIALALR$IGD$FNAYYA-NH2 |
|
| 1217 | Ac-IWIAQALR$IGD$FNAYYA-NH2 |
|
| 1218 | Ac-I$IAA$LRAibIGDAibF$AYY$A-NH2 |
|
| 1219 | Ac-I$IAL$LRAibIGDAibF$AYY$A-NH2 |
|
| 1220 | Ac-I$r8IALALR$IGDAF$AYY$A-NH2 |
|
| 1221 | Ac-I$r8IAQELRStIGDAFN$s8YYARR-NH2 |
|
| 1222 | Ac-I$r8IAQALRStIGDAFN$s8YYA-NH2 |
|
| 1223 | HBS-IAAarALRRIGDEFNAYYAHH-NH2 |
|
| 1224 | HBS-IAAarALR$IGD$FNAYYAHH-NH2 |
|
| 1225 | HBS-IWAarAQALRRIGDEFNAYYAHH-NH2 |
|
| 1226 | HBS-IWAarAQALR$IGD$FNAYYAHH-NH2 |
|
| 1227 | HepIAQ$LRRIGDEFNAYYAHH-NH2 |
|
| 1228 | HepIAQ$LR$IGD$FNAYYAHH-NH2 |
|
| 1229 | HepIA$ALR$IGD$FNAYYAHH-NH2 |
|
| 1230 | Ac-I$IAQ$LR$r8IGDEFN$YYA-NH2 |
|
| 1231 | Ac-I$IAA$LR$r8IGDEFN$YYA-NH2 |
|
| 1232 | Ac-I$IAV$LR$r8IGDEFN$YYA-NH2 |
|
| 1233 | Ac-I$IAV$LR$r8IGDEFN$YYA-NH2 |
|
| 1234 | Ac-I$IAI$LR$r8IGDEFN$YYA-NH2 |
|
| 1235 | Ac-I$IAI$LR$r8IGDEFN$YYA-NH2 |
|
| 1236 | Ac-I$IAY$LR$r8IGDEFN$YYA-NH2 |
|
| 1237 | Ac-I$IAL$LR$r8IGDEFN$YYA-NH2 |
|
| 1238 | Ac-I$IAL$LR$r8IGDEFN$YYA-NH2 |
|
| 1239 | Ac-I$IAF$LR$r8IGDEFN$YYA-NH2 |
|
| 1240 | Ac-I$IAF$LR$r8IGDEFN$YYA-NH2 |
|
| 1241 | Ac-I$IAQ$LR$r8IGDAFN$YYA-NH2 |
|
| 1242 | Ac-I$IAQ$LR$r8IGDNleFN$YYA-NH2 |
|
| 1243 | Ac-I$IAQ$LR$r8IGDAibFN$YYA-NH2 |
|
| 1244 | Ac-I$IAQ$LRRIGDEF$AYY$-NH2 |
|
| 1245 | Ac-I$IAA$LRRIGDEF$AYY$-NH2 |
|
| 1246 | Ac-I$IAV$LRRIGDEF$AYY$-NH2 |
|
| 1247 | Ac-I$IAL$LRRIGDEF$AYY$-NH2 |
|
| 1248 | Ac-I$IAI$LRRIGDEF$AYY$-NH2 |
|
| 1249 | Ac-I$IAF$LRRIGDEF$AYY$-NH2 |
|
| 1250 | Ac-I$IAY$LRRIGDEF$AYY$-NH2 |
|
| 1251 | Ac-I$IAG$LRRIGDEF$AYY$-NH2 |
|
| 1252 | Ac-I$IAQ$LRAIGDAF$AYY$-NH2 |
|
| 1253 | Ac-I$IAQ$LRAIGDAibF$AYY$-NH2 |
|
| 1254 | Ac-I$IAQ$LRAibIGDAF$AYY$-NH2 |
|
| 1255 | Ac-I$IAQ$LRAibIGDAibF$AYY$-NH2 |
|
| 1256 | Ac-I$IAQ$LRNleIGDNleF$AYY$-NH2 |
|
| 1257 | Ac-I$IAQ$LRNleIGDAibF$AYY$-NH2 |
|
| 1258 | Ac-I$IAQ$LRAibIGDNleF$AYY$-NH2 |
|
| 1259 | Ac-IWIA$ALR$IGD$r8FNAYYA$-NH2 |
|
| 1260 | Ac-IWIA$VLR$IGD$r8FNAYYA$-NH2 |
|
| 1261 | Ac-IWIA$LLR$IGD$r8FNAYYA$-NH2 |
|
| 1262 | Ac-IWIA$ILR$IGD$r8FNAYYA$-NH2 |
|
| 1263 | Ac-IWIA$FLR$IGD$r8FNAYYA$-NH2 |
|
| 1264 | Ac-IWIA$YLR$IGD$r8FNAYYA$-NH2 |
|
| 1265 | Ac-IWIA$GLR$IGD$r8FNAYYA$-NH2 |
|
| 1266 | Ac-IWIA$SLR$IGD$r8FNAYYA$-NH2 |
|
| 1267 | Ac-I$r8IALALR$IGDAFN$YYA$A-NH2 |
|
| 1268 | Ac-IWIA$r5ALRStIGDNleFN$r8YYA-NH2 |
|
| 1269 | Ac-I$IAL$LR$r8IGDAFN$YYA-NH2 |
|
| 1270 | Ac-ICou2IAQALR$r5IGDStFNA$YAHH-NH2 |
|
| 1271 | Ac-I$IAQ$LRAIGDAF$AYY$-NH2 |
|
| 1272 | Ac-I$IAQ$LRAIGDAibF$AYY$-NH2 |
|
| 1273 | Ac-I$IAQ$LRAIGDAibF$AYY$-NH2 |
|
| 1274 | Ac-I$IAQ$LRAibIGDAF$AYY$-NH2 |
|
| 1275 | Ac-I$IAQ$LRAibIGDAF$AYY$-NH2 |
|
| 1276 | Ac-I$IAQ$LRAibIGDAibF$AYY$-NH2 |
|
| 1277 | Ac-I$IAQ$LRAibIGDAibF$AYY$-NH2 |
|
| 1278 | Ac-I$IAQ$LRNleIGDNleF$AYY$-NH2 |
|
| 1279 | Ac-I$IAQ$LRNleIGDNleF$AYY$-NH2 |
|
| 1280 | Ac-I$IAQ$LRNleIGDAibF$AYY$-NH2 |
|
| 1281 | Ac-I$IAQ$LRNleIGDAibF$AYY$-NH2 |
|
| 1282 | Ac-IWIA$VLR$IGD$r8FNAYYA$-NH2 |
|
| 1283 | Ac-IWIA$LLR$IGD$r8FNAYYA$-NH2 |
|
| 1284 | Ac-IWIA$FLR$IGD$r8FNAYYA$-NH2 |
|
| 1285 | Ac-IWIA$SLR$IGD$r8FNAYYA$-NH2 |
|
| 1286 | Ac-IWIA$ELR$IGD$r8FNAYYA$-NH2 |
|
| 1287 | Ac-IWIA$ALR$IGD$r8FNAYYA$-NH2 |
|
| 1288 | Ac-I$IAA$LRRIGDEF$AYY$-NH2 |
|
| 1289 | Ac-I$IAA$LRRIGDEF$AYY$-NH2 |
|
| 1290 | Ac-I$IAL$LRRIGDEF$AYY$RR-NH2 |
|
| 1291 | Ac-I$IAQ$LRAibIGDAF$AYY$RR-NH2 |
|
| 1292 | Ac-I$IAL$LRAibIGDAF$AYY$RR-NH2 |
|
| 1293 | Ac-I$IAL$LRRIGDEF$AYY$R-NH2 |
|
| 1294 | Ac-I$IAQ$LRAibIGDAF$AYY$R-NH2 |
|
| 1295 | Ac-I$IAL$LRAibIGDAF$AYY$R-NH2 |
|
| 1296 | Ac-I$IAY$LR$r8IGDEFN$YYARR-NH2 |
|
| 1297 | Ac-I$IAL$LR$r8IGDEFN$YYARR-NH2 |
|
| 1298 | Ac-I$IAF$LR$r8IGDEFN$YYARR-NH2 |
|
| 1299 | Ac-I$IAQ$LR$r8IGDEFN$YYAR-NH2 |
|
| 1300 | Ac-I$IAY$LR$r8IGDEFN$YYAR-NH2 |
|
| 1301 | Ac-I$IAL$LR$r8IGDEFN$YYAR-NH2 |
|
| 1302 | Ac-I$IAF$LR$r8IGDEFN$YYAR-NH2 |
|
| 1303 | Ac-IWIA$ALR$IGD$r8FNAYYA$R-NH2 |
|
| 1304 | Ac-IWIALALR$r8IGDEFN$YYARR-NH2 |
|
| 1305 | Ac-IWIAYALR$r8IGDEFN$YYARR-NH2 |
|
| 1306 | Ac-IWIAQALR$r8IGDEFN$YYAR-NH2 |
|
| 1307 | Ac-IWIALALR$r8IGDEFN$YYAR-NH2 |
|
| 1308 | Ac-IWIAYALR$r8IGDEFN$YYAR-NH2 |
|
| 1309 | Ac-IWIALALR$IGD$FNAYYARR-NH2 |
|
| 1310 | Ac-IWIAYALR$IGD$FNAYYARR-NH2 |
|
| 1311 | Ac-IWIALALR$IGD$FNAYYAR-NH2 |
|
| 1312 | Ac-IWIAYALR$IGD$FNAYYAR-NH2 |
|
| 1313 | Ac-IWIALALR$IGD$FNAYYAH-NH2 |
|
| 1314 | Ac-IWIAQALR%r8IGDAFN%YYA-NH2 |
|
| 1315 | Ac-I$IAL$LRRIGDEF$AYY$RR-NH2 |
|
| 1316 | Ac-I$IAL$LRRIGDEF$AYY$R-NH2 |
|
| 1317 | Ac-I$IAQ$LRAibIGDAF$AYY$R-NH2 |
|
| 1318 | Ac-I$IAL$LRAibIGDAF$AYY$R-NH2 |
|
| 1319 | Ac-I$IAY$LR$r8IGDEFN$YYARR-NH2 |
|
| 1320 | Ac-I$IAL$LR$r8IGDEFN$YYARR-NH2 |
|
| 1321 | Ac-IWIA$ALR$IGD$r8FNAYYA$R-NH2 |
|
| 1322 | Ac-I$IAY$LR$r8IGDEFN$YYAR-NH2 |
|
| 1323 | Ac-I$IAL$LR$r8IGDEFN$YYAR-NH2 |
|
| 1324 | Ac-I$IAF$LR$r8IGDEFN$YYAR-NH2 |
|
| 1325 | Ac-I$IAQ$LR$r8IGDAFN$YYARR-NH2 |
|
| 1326 | Ac-I$IAY$LR$r8IGDAFN$YYARR-NH2 |
|
| 1327 | Ac-I$IAL$LR$r8IGDAFN$YYARR-NH2 |
|
| 1328 | Ac-I$IAF$LR$r8IGDAFN$YYARR-NH2 |
|
| 1329 | Ac-I$IAQ$LR$r8IGDAFN$YYAR-NH2 |
|
| 1330 | Ac-I$IAY$LR$r8IGDAFN$YYAR-NH2 |
|
| 1331 | Ac-I$IAL$LR$r8IGDAFN$YYAR-NH2 |
|
| 1332 | Ac-I$IAF$LR$r8IGDAFN$YYAR-NH2 |
|
| 1333 | Ac-IWIAQALR$r8IGDEFN$YYA-NH2 |
|
| 1334 | Ac-IWIAQALR$r8IGDQFN$YYA-NH2 |
|
| 1335 | Ac-IWIAAALR$r8IGDEFN$YYA-NH2 |
|
| 1336 | Ac-IWIAAALR$r8IGDQFN$YYA-NH2 |
|
| 1337 | Ac-IWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1338 | Ac-IWIAQALR$r8IGDEFA$YYA-NH2 |
|
| 1339 | Ac-IWIAQALR$r8IGDQFA$YYA-NH2 |
|
| 1340 | Ac-IWIAQALR$r8IGDAFA$YYA-NH2 |
|
| 1341 | Ac-IWIAQALCit$r8IGDAFN$YYA-NH2 |
|
| 1342 | Ac-IWIAQALCit$r8IGDQFN$YYA-NH2 |
|
| 1343 | Ac-IWIAQALH$r8IGDAFN$YYA-NH2 |
|
| 1344 | Ac-IWIAQALH$r8IGDQFN$YYA-NH2 |
|
| 1345 | Ac-IWIAQALQ$r8IGDAFN$YYA-NH2 |
|
| 1346 | Ac-IWIAQALQ$r8IGDQFN$YYA-NH2 |
|
| 1347 | Ac-IWIAQALR$r8IGDAAN$YYA-NH2 |
|
| 1348 | Ac-IWIAQALR$r8IGDQAN$YYA-NH2 |
|
| 1349 | Ac-IWIAQALR$r8IGDAIN$YYA-NH2 |
|
| 1350 | Ac-IWIAQALR$r8IGDQIN$YYA-NH2 |
|
| 1351 | Ac-IWIAQAAR$r8IGDAAN$YYA-NH2 |
|
| 1352 | Ac-IWIAQALR$r8IADAFN$YYA-NH2 |
|
| 1353 | Ac-IWIAQALR$r8IADQFN$YYA-NH2 |
|
| 1354 | Ac-IWIAQALR$r8AGDAFN$YYA-NH2 |
|
| 1355 | Ac-IWIAQALR$r8AGDQFN$YYA-NH2 |
|
| 1356 | Ac-IWIAQALR$r8FGDAFN$YYA-NH2 |
|
| 1357 | Ac-IWIAQALR$r8FGDQFN$YYA-NH2 |
|
| 1358 | Ac-IWFAQALR$r8IGDAFN$YYA-NH2 |
|
| 1359 | Ac-IWFAQALR$r8IGDQFN$YYA-NH2 |
|
| 1360 | Ac-IAIAQALR$r8IGDAFN$YYA-NH2 |
|
| 1361 | Ac-IWIAQALA$r8IGDAFN$YYA-NH2 |
|
| 1362 | Ac-IWIAQALR$r8IGNAFN$YYA-NH2 |
|
| 1363 | Ac-IWIAQAAR$r8IGDAFN$YYA-NH2 |
|
| 1364 | FITC-BaIWIAQALR$r8IGDAFN$YYA-NH2 |
|
| 1365 | 5-FAM-BaIWIAQALR$r8IGDAFN$YYA-NH2 |
|
| 1366 | 5-FAM-BaIWIAQALR$r8IGDEFN$YYA-NH2 |
|
| 1367 | Ac-WLAQLLR$IGD$IN-NH2 |
|
| 1368 | Ac-ICou2IALALR$IGD$FNAYYA-NH2 |
|
| 1369 | Ac-ICou2IALALR$IGD$FNAibFYA-NH2 |
|
| 1370 | Ac-I$IAY$LR$r8IGDAFN$YYARR-NH2 |
|
| 1371 | Ac-I$IAL$LR$r8IGDAFN$YYARR-NH2 |
|
| 1372 | Ac-I$IAF$LR$r8IGDAFN$YYARR-NH2 |
|
| 1373 | Ac-I$IAQ$LR$r8IGDAFN$YYAR-NH2 |
|
| 1374 | Ac-I$IAY$LR$r8IGDAFN$YYAR-NH2 |
|
| 1375 | Ac-I$IAL$LR$r8IGDAFN$YYAR-NH2 |
|
| 1376 | Ac-I$IAF$LR$r8IGDAFN$YYAR-NH2 |
|
| 1377 | Ac-IAIAQALR$r8IGDAFN$YYA-NH2 |
|
| 1378 | Ac-IWIAQALR$r8IGDEFN$YYA-NH2 |
|
| 1379 | Ac-IWIAQALR$r8IGDQFN$YYA-NH2 |
|
| 1380 | Ac-IWIAAALR$r8IGDEFN$YYA-NH2 |
|
| 1381 | Ac-IWIAAALR$r8IGDQFN$YYA-NH2 |
|
| 1382 | Ac-IWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1383 | Ac-IWIAQALR$r8IGDAFA$YYA-NH2 |
|
| 1384 | Ac-IWIAQALCit$r8IGDAFN$YYA-NH2 |
|
| 1385 | Ac-IWIAQALCit$r8IGDQFN$YYA-NH2 |
|
| 1386 | Ac-IWIAQALH$r8IGDAFN$YYA-NH2 |
|
| 1387 | Ac-IWIAQALH$r8IGDQFN$YYA-NH2 |
|
| 1388 | Ac-IWIAQALQ$r8IGDAFN$YYA-NH2 |
|
| 1389 | Ac-IWIAQALQ$r8IGDQFN$YYA-NH2 |
|
| 1390 | Ac-IWIAQALR$r8IGDAAN$YYA-NH2 |
|
| 1391 | Ac-IWIAQALR$r8IGDAIN$YYA-NH2 |
|
| 1392 | Ac-IWIAQALR$r8IGDQIN$YYA-NH2 |
|
| 1393 | Ac-IWIAQAAR$r8IGDAAN$YYA-NH2 |
|
| 1394 | Ac-IWIAQALR$r8IADAFN$YYA-NH2 |
|
| 1395 | Ac-IWIAQALR$r8IADQFN$YYA-NH2 |
|
| 1396 | Ac-IWIAQALR$r8AGDAFN$YYA-NH2 |
|
| 1397 | Ac-IWIAQALR$r8AGDQFN$YYA-NH2 |
|
| 1398 | Ac-IWIAQALR$r8FGDAFN$YYA-NH2 |
|
| 1399 | Ac-IWIAQALR$r8FGDQFN$YYA-NH2 |
|
| 1400 | Ac-IWFAQALR$r8IGDAFN$YYA-NH2 |
|
| 1401 | Ac-IWFAQALR$r8IGDQFN$YYA-NH2 |
|
| 1402 | Ac-IWIAQALA$r8IGDAFN$YYA-NH2 |
|
| 1403 | Ac-IWIAQALR$r8IGNAFN$YYA-NH2 |
|
| 1404 | Ac-IWIAQAAR$r8IGDAFN$YYA-NH2 |
|
| 1405 | Ac-IWIALALG$IGD$VNAYYA-NH2 |
|
| 1406 | Ac-IWIALALG$IGD$INAYYA-NH2 |
|
| 1407 | Ac-IWIALALG$IGN$VNAYYA-NH2 |
|
| 1408 | Ac-IWIALALG$IGN$INAYYA-NH2 |
|
| 1409 | Ac-IWIALALN$IGD$VNAYYA-NH2 |
|
| 1410 | Ac-IWIALALN$IGD$INAYYA-NH2 |
|
| 1411 | Ac-IWIALALN$IGN$VNAYYA-NH2 |
|
| 1412 | Ac-IWIALALN$IGN$INAYYA-NH2 |
|
| 1413 | Ac-IWIALALR$IGD$VNAFYA-NH2 |
|
| 1414 | Ac-IWIALALR$IGD$VNAYYA-NH2 |
|
| 1415 | Ac-IWIALALR$IGD$VNAibFYA-NH2 |
|
| 1416 | Ac-IWIALALR$IGD$VNAibYYA-NH2 |
|
| 1417 | Ac-IWFALALR$IGD$FNAYYA-NH2 |
|
| 1418 | Ac-IWYALALR$IGD$FNAYYA-NH2 |
|
| 1419 | Ac-IWVALALR$IGD$FNAYYA-NH2 |
|
| 1420 | Ac-IWLALALR$IGD$FNAYYA-NH2 |
|
| 1421 | Ac-IWIAQALR$IGD$VNAYYA-NH2 |
|
| 1422 | Ac-IWIAQALR$IGD$INAYYA-NH2 |
|
| 1423 | Ac-IWIALALR$IGD$INAYYA-NH2 |
|
| 1424 | Ac-IWIALLLR$IGD$VNAYYA-NH2 |
|
| 1425 | Ac-IWIALLLR$IGD$INAYYA-NH2 |
|
| 1426 | Ac-IWIALALG$IGD$FNAYYA-NH2 |
|
| 1427 | Ac-IWIALALS$IGD$FNAYYA-NH2 |
|
| 1428 | Ac-IWIALALH$IGD$FNAYYA-NH2 |
|
| 1429 | Ac-IWIALALN$IGD$FNAYYA-NH2 |
|
| 1430 | Ac-IWIALAIG$IGD$VNAYYA-NH2 |
|
| 1431 | Ac-IWIALAIG$IGD$INAYYA-NH2 |
|
| 1432 | Ac-IWIALAIN$IGD$VNAYYA-NH2 |
|
| 1433 | Ac-IWIALAIN$IGD$INAYYA-NH2 |
|
| 1434 | Ac-IWIALALN$IGD$VNAYYAHH-NH2 |
|
| 1435 | Ac-IWIALALN$IGD$INAYYAHH-NH2 |
|
| 1436 | Ac-IWIALALN$IGN$VNAYYAHH-NH2 |
|
| 1437 | Ac-IWIALALN$IGN$INAYYAHH-NH2 |
|
| 1438 | Ac-IWIA$r5ALGStIGD$VNAYYA-NH2 |
|
| 1439 | Ac-IWIA$r5ALGStIGD$INAYYA-NH2 |
|
| 1440 | Ac-IWIA$r5ALGStIGN$VNAYYA-NH2 |
|
| 1441 | Ac-IWIA$r5ALGStIGN$INAYYA-NH2 |
|
| 1442 | Ac-IWIALALR$IGD$VNAAAA-NH2 |
|
| 1443 | Ac-IWIALALG$IGD$VNAAAA-NH2 |
|
| 1444 | Ac-IWIALALD$IGD$VNAAAA-NH2 |
|
| 1445 | Ac-IWIALALN$IGD$VNAAAA-NH2 |
|
| 1446 | Ac-IWIALALR$IGD$VN-NH2 |
|
| 1447 | Ac-IWIALALG$IGD$VN-NH2 |
|
| 1448 | Ac-IWIALALD$IGD$VN-NH2 |
|
| 1449 | Ac-IWIALALN$IGD$VN-NH2 |
|
| 1450 | 5-FAM-BaIWIA$r5ALGStIGD$VNAYYA-NH2 |
|
| 1451 | 5-FAM-BaIWIALALR$IGD$FNAibFYA-NH2 |
|
| 1452 | 5-FAM-BaIWIA$r5ALGStIGN$INAYYA-NH2 |
|
| 1453 | 5-FAM-BaIWIALALG$IGN$INAYYA-NH2 |
|
| 1454 | FITC-BaIWIA$r5ALGStIGD$VNAYYA-NH2 |
|
| 1455 | FITC-BaIWIALALR$IGD$FNAibFYA-NH2 |
|
| 1456 | 5-FAM-BaIWIA$r5ALGStIGD$INAYYA-NH2 |
|
| 1457 | Ac-IWIAQALR$r8IGDQFA$YYA-NH2 |
|
| 1458 | Ac-RWIAQALR$IGD$LNAFYAHH-NH2 |
|
| 1459 | Ac-RWIAQELR$IGD$LNAibFYAHH-NH2 |
|
| 1460 | Ac-RWIAQALR$IGD$LNAibFYA-NH2 |
|
| 1461 | Ac-RWIAQAAR$IGD$LNAibFYAHH-NH2 |
|
| 1462 | Ac-RWIAQALA$IGD$LNAibFYAHH-NH2 |
|
| 1463 | Ac-RWIAQALR$IGN$LNAibFYAHH-NH2 |
|
| 1464 | Ac-RWIAQALCit$IGD$LNAibFYAHH-NH2 |
|
| 1465 | Ac-RWIAQALR$IGD$ANAibFYAHH-NH2 |
|
| 1466 | Ac-RCou2IAQAAR$IGD$LNAibFYAHH-NH2 |
|
| 1467 | Ac-RCou2IAQALA$IGD$LNAibFYAHH-NH2 |
|
| 1468 | Ac-RCou2IAQALR$IGN$LNAibFYAHH-NH2 |
|
| 1469 | Ac-RCou2IAQALCit$IGD$LNAibFYAHH-NH2 |
|
| 1470 | Ac-IWIAMOALCit$r8IGDAFN$YYA-NH2 |
|
| 1471 | Ac-IWIAMO2ALCit$r8IGDAFN$YYA-NH2 |
|
| 1472 | Ac-RWIAMOALR$IGD$LNAibFYAHH-NH2 |
|
| 1473 | Ac-RWIAMO2ALR$IGD$LNAibFYAHH-NH2 |
|
| 1474 | Ac-RWIAQALR$IGN$VNAibFYAHH-NH2 |
|
| 1475 | Ac-RWIAQAAR$IGD$VNAibFYAHH-NH2 |
|
| 1476 | Ac-RWIAQALA$IGD$VNAibFYAHH-NH2 |
|
| 1477 | Ac-RWIAQALCit$IGD$VNAibFYAHH-NH2 |
|
| 1478 | Ac-RCou2IAQALR$IGD$VNAibFYAHH-NH2 |
|
| 1479 | Ac-RCou2IAQALR$IGN$VNAibFYAHH-NH2 |
|
| 1480 | Ac-RCou2IAQAAR$IGD$VNAibFYAHH-NH2 |
|
| 1481 | Ac-RCou2IAQALA$IGD$VNAibFYAHH-NH2 |
|
| 1482 | Ac-RCou2IAQALCit$IGD$VNAibFYAHH-NH2 |
|
| 1483 | Ac-IWChaAQALR$r8IGDAFN$YYA-NH2 |
|
| 1484 | Ac-IWhhLAQALR$r8IGDAFN$YYA-NH2 |
|
| 1485 | Ac-IWAdmAQALR$r8IGDAFN$YYA-NH2 |
|
| 1486 | Ac-IWhChaAQALR$r8IGDAFN$YYA-NH2 |
|
| 1487 | Ac-IWhFAQALR$r8IGDAFN$YYA-NH2 |
|
| 1488 | Ac-IWIglAQALR$r8IGDAFN$YYA-NH2 |
|
| 1489 | Ac-IWF4CF3AQALR$r8IGDAFN$YYA-NH2 |
|
| 1490 | Ac-IWF4tBuAQALR$r8IGDAFN$YYA-NH2 |
|
| 1491 | Ac-IW2NalAQALR$r8IGDAFN$YYA-NH2 |
|
| 1492 | Ac-IWBipAQALR$r8IGDAFN$YYA-NH2 |
|
| 1493 | Ac-IWIAQAChaR$r8IGDAFN$YYA-NH2 |
|
| 1494 | Ac-IWIAQAhhLR$r8IGDAFN$YYA-NH2 |
|
| 1495 | Ac-IWIAQAAdmR$r8IGDAFN$YYA-NH2 |
|
| 1496 | Ac-IWIAQAhChaR$r8IGDAFN$YYA-NH2 |
|
| 1497 | Ac-IWIAQAhAdmR$r8IGDAFN$YYA-NH2 |
|
| 1498 | Ac-IWIAQAhFR$r8IGDAFN$YYA-NH2 |
|
| 1499 | Ac-IWIAQAIglR$r8IGDAFN$YYA-NH2 |
|
| 1500 | Ac-IWIAQAF4CF3R$r8IGDAFN$YYA-NH2 |
|
| 1501 | Ac-IWIAQAF4tBuR$r8IGDAFN$YYA-NH2 |
|
| 1502 | Ac-IWIAQA2NalR$r8IGDAFN$YYA-NH2 |
|
| 1503 | Ac-IWIAQABipR$r8IGDAFN$YYA-NH2 |
|
| 1504 | Ac-IWIAQALR$r8CbaGDAFN$YYA-NH2 |
|
| 1505 | Ac-IWIAQALR$r8hLGDAFN$YYA-NH2 |
|
| 1506 | Ac-IWIAQALR$r8ChaGDAFN$YYA-NH2 |
|
| 1507 | Ac-IWIAQALR$r8TbaGDAFN$YYA-NH2 |
|
| 1508 | Ac-IWIAQALR$r8hhLGDAFN$YYA-NH2 |
|
| 1509 | Ac-IAmWIAQALR$r8IGDAFN$YYA-NH2 |
|
| 1510 | Ac-IAibIAQALR$r8IGDAFN$YYA-NH2 |
|
| 1511 | Ac-AmLWIAQALR$r8IGDAFN$YYA-NH2 |
|
| 1512 | Ac-IWAmLAQALR$r8IGDAFN$YYA-NH2 |
|
| 1513 | Ac-IWIAibQALR$r8IGAmDAFN$YYA-NH2 |
|
| 1514 | Ac-IWIAAibALR$r8IGDAFN$YYA-NH2 |
|
| 1515 | Ac-IWIAQAAmLR$r8IGDAFN$YYA-NH2 |
|
| 1516 | Ac-IWIAQALR$r8IGAmDAFN$YYA-NH2 |
|
| 1517 | Ac-IWIAQALR$r8IGDAFN$F4FYA-NH2 |
|
| 1518 | Ac-IWIAQALR$r8IGDAFN$AYA-NH2 |
|
| 1519 | Ac-IWIAQALR$r8IGDAFN$YF4FA-NH2 |
|
| 1520 | Ac-IWIAQALR$r8IGDAFN$YYAib-NH2 |
|
| 1521 | Ac-I$r8IAQALRStIGDEFN$s8YYA-NH2 |
|
| 1522 | Ac-IWIA$ALRStIGDEFN$s8YYA-NH2 |
|
| 1523 | Ac-IWIAQALR$r8IGDEFNStYYA$r5A-NH2 |
|
| 1524 | Ac-IWIAQAACit$r8IGDAFN$YYA-NH2 |
|
| 1525 | Ac-IWIAQALCit$r8IGNAFN$YYA-NH2 |
|
| 1526 | Ac-IWIAQALCit$r8IGDAAN$YYA-NH2 |
|
| 1527 | Ac-IWIAQALCit$r8IGDAVN$YYA-NH2 |
|
| 1528 | Ac-RWIAQAChaR$IGD$LNAibFYAHH-NH2 |
|
| 1529 | Ac-RWIAQAhhLR$IGD$LNAibFYAHH-NH2 |
|
| 1530 | Ac-RWIAQAAdmR$IGD$LNAibFYAHH-NH2 |
|
| 1531 | Ac-RWIAQAhChaR$IGD$LNAibFYAHH-NH2 |
|
| 1532 | Ac-RWIAQAhFR$IGD$LNAibFYAHH-NH2 |
|
| 1533 | Ac-RWIAQAIglR$IGD$LNAibFYAHH-NH2 |
|
| 1534 | Ac-RWIAQAF4CF3R$IGD$LNAibFYAHH-NH2 |
|
| 1535 | Ac-RWIAQAF4tBuR$IGD$LNAibFYAHH-NH2 |
|
| 1536 | Ac-RWIAQA2NalR$IGD$LNAibFYAHH-NH2 |
|
| 1537 | Ac-RWIAQABipR$IGD$LNAibFYAHH-NH2 |
|
| 1538 | Ac-IWIAQ$r8LRRIGD$FNAYYA-NH2 |
|
| 1539 | Ac-IWIAQ$r8LRAIGD$FNAYYA-NH2 |
|
| 1540 | Ac-IWIAQ$r8LCitRIGD$FNAYYA-NH2 |
|
| 1541 | Ac-IWIAQ$r8LCitAIGD$FNAYYA-NH2 |
|
| 1542 | Ac-IWIAMOALCit$r8IGDAFN$YYA-NH2 |
|
| 1543 | Ac-IWIAMO2ALCit$r8IGDAFN$YYA-NH2 |
|
| 1544 | Ac-IWIAQALD$r8IGRAFN$YYA-NH2 |
|
| 1545 | Ac-RWIAQALD$IGR$LNAibFYAHH-NH2 |
|
| 1546 | Ac-RPEIWIAQAID$r8IGDAVN$YYAR-NH2 |
|
| 1547 | Ac-RPEIWIAQAID$IGD$VNAYYAR-NH2 |
|
| 1548 | Ac-DWIAQALR$r8IGDAFN$YYR-NH2 |
|
| 1549 | Ac-IWAAQALR$r8IGDAFN$YYA-NH2 |
|
| 1550 | Ac-IWTbaAQALR$r8IGDAFN$YYA-NH2 |
|
| 1551 | Ac-IWhLAQALR$r8IGDAFN$YYA-NH2 |
|
| 1552 | Ac-IWChgAQALR$r8IGDAFN$YYA-NH2 |
|
| 1553 | Ac-IWAc6cAQALR$r8IGDAFN$YYA-NH2 |
|
| 1554 | Ac-IWAc5cAQALR$r8IGDAFN$YYA-NH2 |
|
| 1555 | Ac-EWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1556 | Ac-RWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1557 | Ac-KWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1558 | Ac-HWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1559 | Ac-SWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1560 | Ac-QWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1561 | Ac-AWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1562 | Ac-AibWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1563 | Ac-FWIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1564 | Ac-IDIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1565 | Ac-IRIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1566 | Ac-IHIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1567 | Ac-ISIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1568 | Ac-INIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1569 | Ac-ILIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1570 | Ac-IFIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1571 | Ac-I2NalIAAALR$r8IGDAFN$YYA-NH2 |
|
| 1572 | Ac-IWISAALR$r8IGDAFN$YYA-NH2 |
|
| 1573 | Ac-IWILAALR$r8IGDAFN$YYA-NH2 |
|
| 1574 | Ac-IWIFAALR$r8IGDAFN$YYA-NH2 |
|
| 1575 | Ac-IWIALALR$r8IGDAFN$YYA-NH2 |
|
| 1576 | Ac-IWIAAALF4g$r8IGDAFN$YYA-NH2 |
|
| 1577 | Ac-IWIAAALK$r8IGDAFN$YYA-NH2 |
|
| 1578 | Ac-IWIAAALR$r8IAbuDAFN$YYA-NH2 |
|
| 1579 | Ac-IWIAAALR$r8IVDAFN$YYA-NH2 |
|
| 1580 | Ac-IWIAAALR$r8IGEAFN$YYA-NH2 |
|
| 1581 | Ac-IWIAAALR$r8IGDAGN$YYA-NH2 |
|
| 1582 | Ac-IWIAQALR$r8IGDAWN$YYA-NH2 |
|
| 1583 | Ac-IWIAQALR$r8IGDAhFN$YYA-NH2 |
|
| 1584 | Ac-IWIAQALR$r8IGDAF4CF3N$YYA-NH2 |
|
| 1585 | Ac-IWIAQALR$r8IGDAF4tBuN$YYA-NH2 |
|
| 1586 | Ac-IWIAQALR$r8IGDA2NalN$YYA-NH2 |
|
| 1587 | Ac-IWIAQALR$r8IGDABipN$YYA-NH2 |
|
| 1588 | Ac-IWIAAALR$r8IGDAFD$YYA-NH2 |
|
| 1589 | Ac-IWIAAALR$r8IGDAFE$YYA-NH2 |
|
| 1590 | Ac-IWIAAALR$r8IGDAFQ$YYA-NH2 |
|
| 1591 | Ac-IWIAAALR$r8IGDAFS$YYA-NH2 |
|
| 1592 | Ac-IWIAAALR$r8IGDAFH$YYA-NH2 |
|
| 1593 | Ac-IWIAAALR$r8IGDAFN$LYA-NH2 |
|
| 1594 | Ac-IWIAQALR$r8IGDAFN$YAA-NH2 |
|
| 1595 | Ac-IWIAQALR$r8IGDAFN$YLA-NH2 |
|
| 1596 | Ac-IWIAQALR$r8IGDAFN$YChaA-NH2 |
|
| 1597 | Ac-IWIAQALR$r8IGDAFN$YhFA-NH2 |
|
| 1598 | Ac-IWIAQALR$r8IGDAFN$YWA-NH2 |
|
| 1599 | Ac-IWIAQALR$r8IGDAFN$Y2NalA-NH2 |
|
| 1600 | Ac-IWIAAALR$r8IGDAFN$YYD-NH2 |
|
| 1601 | Ac-IWIAAALR$r8IGDAFN$YYE-NH2 |
|
| 1602 | Ac-IWIAAALR$r8IGDAFN$YYQ-NH2 |
|
| 1603 | Ac-IWIAAALR$r8IGDAFN$YYS-NH2 |
|
| 1604 | Ac-IWIAAALR$r8IGDAFN$YYH-NH2 |
|
| 1605 | Ac-IWIAAALR$r8IGDAFN$YYR-NH2 |
|
| 1606 | Ac-IWIAAALR$r8IGDAFN$YYK-NH2 |
|
| 1607 | Ac-IWIAQALR$rda6IGDAFN$da5YYA-NH2 |
|
| 1608 | Ac-IWIAQAAmLR$r8IGDAFN$YYA-NH2 |
|
| 1609 | Ac-IWIAQALR$r8IGAmDAFN$YYA-NH2 |
|
| 1610 | Ac-IWIAQALR$r8IGDAFN$F4FYA-NH2 |
|
| 1611 | Ac-IWIAQALR$r8IGDAFN$YYAib-NH2 |
|
| 1612 | Ac-IWIAQAACit$r8IGDAFN$YYA-NH2 |
|
| 1613 | Ac-IWIAQALCit$r8IGNAFN$YYA-NH2 |
|
| 1614 | Ac-IWIAQALCit$r8IGDAAN$YYA-NH2 |
|
| 1615 | Ac-IWIAQALCit$r8IGDAVN$YYA-NH2 |
|
| 1616 | Ac-IWIAQ$r8LRAIGD$FNAYYA-NH2 |
|
| 1617 | Ac-IWIAQ$r8LCitAIGD$FNAYYA-NH2 |
|
| 1618 | Ac-IWIAQALR$r8IGDAFN$AYA-NH2 |
|
| 1619 | Ac-IWIAQ$r8LRRIGD$FNAYYA-NH2 |
|
| 1620 | Ac-IWIAQALR$r8hLGDAFN$F4FYA-NH2 |
|
| 1621 | Ac-IWIAQALR$r8hLGDAFN$YF4FA-NH2 |
|
| 1622 | Ac-IWIAQALR$r8hLGDAFN$F4FF4FA-NH2 |
|
| 1623 | Ac-AWIAAALR$r8hLGDAFN$YF4FA-NH2 |
|
| 1624 | Ac-AWIAAALR$r8hLGDAFN$AF4FA-NH2 |
|
| 1625 | Ac-IWIAQAAR$r8hLGDAFN$F4FF4FA-NH2 |
|
[0120]In the sequences shown above and elsewhere, the following abbreviations are used: “Nle” represents norleucine, “Aib” represents 2-aminoisobutyric acid, “Ac” represents acetyl, and “Pr” represents propionyl. Amino acids represented as “$” are alpha-Me S5-pentenyl-alanine olefin amino acids connected by an all-carbon crosslinker comprising one double bond. Amino acids represented as “$r5” are alpha-Me R5-pentenyl-alanine olefin amino acids connected by an all-carbon comprising one double bond. Amino acids represented as “$s8” are alpha-Me S8-octenyl-alanine olefin amino acids connected by an all-carbon crosslinker comprising one double bond. Amino acids represented as “$r8” are alpha-Me R8-octenyl-alanine olefin amino acids connected by an all-carbon crosslinker comprising one double bond. “Ahx” represents an aminocyclohexyl linker. The crosslinkers are linear all-carbon crosslinker comprising eight or eleven carbon atoms between the alpha carbons of each amino acid. Amino acids represented as “$/” are alpha-Me S5-pentenyl-alanine olefin amino acids that are not connected by any crosslinker. Amino acids represented as “$/r5” are alpha-Me R5-pentenyl-alanine olefin amino acids that are not connected by any crosslinker. Amino acids represented as “$/s8” are alpha-Me S8-octenyl-alanine olefin amino acids that are not connected by any crosslinker. Amino acids represented as “$/r8” are alpha-Me R8-octenyl-alanine olefin amino acids that are not connected by any crosslinker. Amino acids represented as “Amw” are alpha-Me tryptophan amino acids. Amino acids represented as “Aml” are alpha-Me leucine amino acids. Amino acids represented as “Amf” are alpha-Me phenylalanine amino acids. Amino acids represented as “2ff” are 2-fluoro-phenylalanine amino acids. Amino acids represented as “3ff” are 3-fluoro-phenylalanine amino acids. Amino acids represented as “St” are amino acids comprising two pentenyl-alanine olefin side chains, each of which is crosslinked to another amino acid as indicated. Amino acids represented as “St//” are amino acids comprising two pentenyl-alanine olefin side chains that are not crosslinked. Amino acids represented as “% St” are amino acids comprising two pentenyl-alanine olefin side chains, each of which is crosslinked to another amino acid as indicated via fully saturated hydrocarbon crosslinks. Amino acids represented as “Ba” are beta-alanine. The lower-case character “e” or “z” within the designation of a crosslinked amino acid (e.g., “$er8” or “$zr8”) represents the configuration of the double bond (E or Z, respectively). In other contexts, lower-case letters such as “a” or “f” represent D amino acids (e.g., D-alanine, or D-phenylalanine, respectively). Amino acids designated as “NmW” represent N-methyltryptophan. Amino acids designated as “NmY” represent N-methyltyrosine. Amino acids designated as “NmA” represent N-methylalanine. “Kbio” represents a biotin group attached to the side chain amino group of a lysine residue. Amino acids designated as “Sar” represent sarcosine. Amino acids designated as “Cha” represent cyclohexyl alanine. Amino acids designated as “Cpg” represent cyclopentyl glycine. Amino acids designated as “Chg” represent cyclohexyl glycine. Amino acids designated as “Cba” represent cyclobutyl alanine. Amino acids designated as “F4I” represent 4-iodo phenylalanine. “7L” represents N15 isotopic leucine. Amino acids designated as “F3Cl” represent 3-chloro phenylalanine. Amino acids designated as “F4cooh” represent 4-carboxy phenylalanine. Amino acids designated as “F34F2” represent 3,4-difluoro phenylalanine. Amino acids designated as “6clW” represent 6-chloro tryptophan. Amino acids designated as “$rda6” represent alpha-Me R6-hexynyl-alanine alkynyl amino acids, crosslinked via a dialkyne bond to a second alkynyl amino acid. Amino acids designated as “$da5” represent alpha-Me S5-pentynyl-alanine alkynyl amino acids, wherein the alkyne forms one half of a dialkyne bond with a second alkynyl amino acid. Amino acids designated as “$ra9” represent alpha-Me R9-nonynyl-alanine alkynyl amino acids, crosslinked via an alkyne metathesis reaction with a second alkynyl amino acid. Amino acids designated as “$a6” represent alpha-Me S6-hexynyl-alanine alkynyl amino acids, crosslinked via an alkyne metathesis reaction with a second alkynyl amino acid. The designation “iso1” or “iso2” indicates that the peptidomimetic macrocycle is a single isomer. Amino acids designated as “Cit” represent citrulline.
[0121]Amino acids which are used in the formation of triazole crosslinkers are represented according to the legend indicated below. Stereochemistry at the alpha position of each amino acid is S unless otherwise indicated. For azide amino acids, the number of carbon atoms indicated refers to the number of methylene units between the alpha carbon and the terminal azide. For alkyne amino acids, the number of carbon atoms indicated is the number of methylene units between the alpha position and the triazole moiety plus the two carbon atoms within the triazole group derived from the alkyne.
[0000] | $5a5 | Alpha-Me alkyne 1,5 triazole (5 carbon) |
| $5n3 | Alpha-Me azide 1,5 triazole (3 carbon) |
| $4rn6 | Alpha-Me R-azide 1,4 triazole (6 carbon) |
| $4a5 | Alpha-Me alkyne 1,4 triazole (5 carbon) |
| |
[0122]In some embodiments, peptidomimetic macrocycles are provided which are derived from BIM. In some embodiments, the present invention provides a peptidomimetic macrocycle comprising an amino acid sequence which is at least about 60% identical to BIM, further comprising at least two macrocycle-forming linkers, wherein the first of said two macrocycle-forming linkers connects a first amino acid to a second amino acid, and the second of said two macrocycle-forming linkers connects a third amino acid to a fourth amino acid.
[0123]Two or more peptides can share a degree of homology. In some embodiments, the pair of peptides is a peptidomimetic macrocycle of the present disclosure and a peptide identical to BIM. A pair of peptides can have, for example, up to about 20% pairwise homology, up to about 25% pairwise homology, up to about 30% pairwise homology, up to about 35% pairwise homology, up to about 40% pairwise homology, up to about 45% pairwise homology, up to about 50% pairwise homology, up to about 55% pairwise homology, up to about 60% pairwise homology, up to about 65% pairwise homology, up to about 70% pairwise homology, up to about 75% pairwise homology, up to about 80% pairwise homology, up to about 85% pairwise homology, up to about 90% pairwise homology, up to about 95% pairwise homology, up to about 96% pairwise homology, up to about 97% pairwise homology, up to about 98% pairwise homology, up to about 99% pairwise homology, up to about 99.5% pairwise homology, or up to about 99.9% pairwise homology. A pair of peptides can have, for example, at least about 20% pairwise homology, at least about 25% pairwise homology, at least about 30% pairwise homology, at least about 35% pairwise homology, at least about 40% pairwise homology, at least about 45% pairwise homology, at least about 50% pairwise homology, at least about 55% pairwise homology, at least about 60% pairwise homology, at least about 65% pairwise homology, at least about 70% pairwise homology, at least about 75% pairwise homology, at least about 80% pairwise homology, at least about 85% pairwise homology, at least about 90% pairwise homology, at least about 95% pairwise homology, at least about 96% pairwise homology, at least about 97% pairwise homology, at least about 98% pairwise homology, at least about 99% pairwise homology, at least about 99.5% pairwise homology, at least about 99.9% pairwise homology.
[0124]Various methods and software programs can be used to determine the homology between two or more peptides, such as NCBI BLAST, Clustal W, MAFFT, Clustal Omega, AlignMe, Praline, or another suitable method or algorithm.
[0125]In some embodiments, a peptidomimetic macrocycle of the invention comprises a helix, for example an α-helix. In some embodiments, a peptidomimetic macrocycle of the invention comprises an α,α-disubstituted amino acid. In some embodiments, each amino acid connected by the macrocycle-forming linker is an α,α-disubstituted amino acid.
[0126]In some embodiments, a peptidomimetic macrocycle of the invention has the Formula (I):
[0000]
[0127]each A, C, D, and E is independently an amino acid (including natural or non-natural amino acids and amino acid analogues) and the terminal D and E independently optionally include a capping group;
[0128]each B is independently an amino acid (including natural or non-natural amino acids and amino acid analogues),
[0000]
[0000][—NH-L3-CO—], [—NH-L3-SO2—], or [—NH-L3-];
[0129]each R1and R2is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, unsubstituted or substituted with halo-; or at least one of R1and R2forms a macrocycle-forming linker L′ connected to the alpha position of one of said D or E amino acids;
[0130]each R3is independently hydrogen, alkyl, alkenyl, alkynyl, arylalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, cycloalkylalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5;
- each L and L′ is independently a macrocycle-forming linker of the formula -L1-L2-,
[0000]
[0132]each L1, L2and L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, cycloarylene, heterocycloarylene, or [—R4—K—R4-]n, each being optionally substituted with R5; when L is not
[0000]
[0000]or -L1-S-L2-S-L3-, L1and L2are alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, cycloarylene, or heterocycloarylene;
[0133]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene;
[0134]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0135]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0136]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0137]each R7is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5, or part of a cyclic structure with a D residue;
[0138]each R8is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5, or part of a cyclic structure with an E residue;
[0139]each R9is independently absent, hydrogen, alkyl, alkenyl, alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, or heterocyclyl group, unsubstituted or optionally substituted with Raor Rb;
[0140]each Raand Rbis independently alkyl, OCH3, CF3, NH2, CH2NH2, F, Br, I,
[0000]
[0141]each v and w is independently an integer from 0-1000, for example 0-500, 0-200, 0-100, 0-50, 0-30, 0-20, or 0-10;
[0142]u is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, for example 1-5, 1-3 or 1-2;
[0143]each x, y and z is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, for example the sum of x+y+z is 2, 3, or 6;
[0144]each n is independently 1, 2, 3, 4, or 5; and
[0000]wherein A, B, C, D, and E, taken together with the crosslinked amino acids connected by the macrocycle-forming linker, -L1-L2-, form an amino acid sequence of the peptidomimetic macrocycle which is at least about 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, or 99% identical to BIM 1-44, BIM 1-29 or to an amino acid sequence chosen from the group consisting of the amino acid sequences in Table 1;
[0145]In some embodiments, u is 1.
[0146]In some embodiments, the sum of x+y+z is 2, 3, 6, or 10, for example 2, 3 or 6, for example 3 or 6.
[0147]In some embodiments, the sum of x+y+z is 3.
[0148]In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10. In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15. In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20. In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25.
[0149]In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15.
[0150]In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30. In some embodiments, w is an integer from 3-1000, for example 3-500, 3-200, 3-100, 3-50, 3-30, 3-20, or 3-10. In some embodiments, the sum of x+y+z is 3 or 6. In some embodiments, the sum of x+y+z is 3. In other embodiments, the sum of x+y+z is 6.
[0151]In some embodiments, w is 3, 4, 5, 6, 7, 8, 9, or 10. In some embodiments, w is 3, 4, 5, or 6. In some embodiments, w is 3, 4, 5, 6, 7, or 8. In some embodiments, w is 6, 7, 8, 9, or 10. In some embodiments, w is 3. In other embodiments, w is 6. In some embodiments, v is an integer from 1-1000, for example 1-500, 1-200, 1-100, 1-50, 1-30, 1-20, or 1-10. In some embodiments, v is 2.
[0152]In some embodiments, L1and L2are independently alkylene, alkenylene or alkynylene.
[0153]In some embodiments, L1and L2are independently C3-C10alkylene or alkenylene.
[0154]In some embodiments, L1and L2are independently C3-C6alkylene or alkenylene.
[0155]In some embodiments, L or L′ is:
[0000]
[0156]In some embodiments, L or L′ is
[0000]
[0157]For example, L or L′ is
[0000]
[0158]In some embodiments, R1and R2are H.
[0159]In some embodiments, R1and R2are independently alkyl.
[0160]In some embodiments, R1and R2are methyl.
[0161]In some embodiments, the present invention provides a peptidomimetic macrocycle having the Formula (Ia):
[0000]
[0162]R8′ is —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5, or part of a cyclic structure with a E residue;
[0163]v′ and w′ are independently integers from 0-100; and
[0164]x′, y′ and z′ are independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, for example, x′+y′+z′ is 2, 3, 6 or 10.
[0165]In some embodiments, u is 2.
[0166]In some embodiments, the peptidomimetic macrocycle of Formula (I) has the Formula (Ib):
[0000]
[0167]R7′ is —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5, or part of a cyclic structure with a D residue;
[0168]R8′ is —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5, or part of a cyclic structure with an E residue;
[0169]v′ and w′ are independently integers from 0-100; and
[0170]x′, y′ and z′ are independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10.
[0171]In some embodiments, the sum of x+y+z is 2, 3 or 6, for example 3 or 6.
[0172]In some embodiments, the sum of x′+y′+z′ is 2, 3 or 6, for example 3 or 6.
[0173]In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10. In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15. In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, or 20. In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, or 25.
[0174]In some embodiments, each v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30. In some embodiments, w is an integer from 3-1000, for example 3-500, 3-200, 3-100, 3-50, 3-30, 3-20, or 3-10. In some embodiments, the sum of x+y+z is 3 or 6. In some embodiments, the sum of x+y+z is 3. In other embodiments, the sum of x+y+z is 6.
[0175]In some embodiments, w is 3, 4, 5, 6, 7, 8, 9, or 10. In some embodiments, w is 3, 4, 5, or 6. In some embodiments, w is 3, 4, 5, 6, 7, or 8. In some embodiments, w is 6, 7, or 8. In some embodiments, w is 6, 7, 8, 9, or 10. In some embodiments, w is 3. In other embodiments, w is 6. In some embodiments, v is an integer from 1-1000, for example 1-500, 1-200, 1-100, 1-50, 1-30, 1-20, or 1-10. In some embodiments, v is 2.
[0176]In some embodiments, a peptidomimetic macrocycle of the invention comprises an amino acid sequence which is at least about 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, or 99% identical to an amino acid sequence of Table 1, and comprising at least one macrocycle-forming linker, wherein the macrocycle-forming linker connects amino acids 14 and 18.
[0177]In some embodiments, a peptidomimetic macrocycle of Formula (I) has Formula (Ic):
[0000]
[0178]each A, C, D, and E is independently a natural or non-natural amino acid;
[0179]each B is independently a natural or non-natural amino acid, amino acid analogue,
[0000]
[0000][—NH-L3-CO—], [—NH-L3-SO2—], or [—NH-L3-];
[0180]each L is independently a macrocycle-forming linker;
[0181]each L′ is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5, or a bond, or together with R1and the atom to which both R1and L′ are bound forms a ring;
[0182]each L″ is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5, or a bond, or together with R2and the atom to which both R2and L″ are bound forms a ring;
[0183]each R1is independently-H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, unsubstituted or substituted with halo-, or together with L′ and the atom to which both R1and L′ are bound forms a ring;
[0184]each R2is independently-H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, unsubstituted or substituted with halo-, or together with L″ and the atom to which both R2and L″ are bound forms a ring;
[0185]each R3is independently hydrogen, alkyl, alkenyl, alkynyl, arylalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, cycloalkylalkyl, aryl, or heteroaryl, optionally substituted with R5;
[0186]each L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, heteroarylene, or [—R4—K—R4-]n, each being optionally substituted with R5;
[0187]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene;
[0188]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0189]n is 1, 2, 3, 4, or 5;
[0190]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0191]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0192]each R7is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, optionally substituted with R5, or part of a cyclic structure with a D residue;
[0193]each R8is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, optionally substituted with R5, or part of a cyclic structure with an E residue;
[0194]each v and w is independently an integer from 1-1000, for example 1-500, 1-200, 1-100, 1-50, 1-40, 1-25, 1-20, 1-15, or 1-10;
[0195]u is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; and
[0196]each x, y and z is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10.
[0197]In some embodiments, the peptidomimetic macrocycle comprises two crosslinks, wherein a first crosslink is of a first pair of amino acid residues, and a second crosslink is of a second pair of amino acid residues. In some embodiments, the first pair of amino acid residues and the second pair of amino acid residues do not share a common amino acid residue. In some embodiments, the first pair of amino acid residues and the second pair of amino acid residues share one common amino acid residue.
[0198]In some embodiments, w is at least about 1, at least about 2, at least about 3, at least about 4, at least about 5, at least about 6, at least about 7, at least about 8, at least about 9, or at least about 10. In some embodiments, w is from about 1 to about 2, from about 2 to about 3, from about 3 to about 4, from about 4 to about 5, from about 5 to about 6, from about 6 to about 7, from about 7 to about 8, from about 8 to about 9, or from about 9 to about 10.
[0199]In some embodiments, w is at least 2 and at least one of the last two E residues is a His residue. In some embodiments, w is at least 2 and at least one of the last two E residues is an Arg residue. In some embodiments, w is at least 2 and both of the last two E residues are His residues. In some embodiments, w is at least 2 and both of the last two E residues are Arg residues. The number of His residues at the peptide C-terminus, or at the E variable, can be 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10. The His residues can be contiguous, or interrupted by a gap of i, i+1, i+2, i+3, or i+4.
[0200]In some embodiments, the peptidomimetic macrocycle comprises a helix. In some embodiments, the peptidomimetic macrocycle comprises an α-helix. In some embodiments, each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15. In some embodiments, each of v and w is independently 3, 4, 5, 6, 7, 8, 9, or 10. In some embodiments, v is 8. In some embodiments, w is 6. In some embodiments, the crosslinked amino acid residues are at positions 9 and 13 of the peptidomimetic macrocycle.
[0201]In some embodiments, L is
[0000]
[0202]In some embodiments, R1and R2are H. In some embodiments, R1and R2are independently alkyl. In some embodiments, R1and R2are methyl.
[0203]In some embodiments, the peptidomimetic macrocycles have the Formula (I):
[0000]
[0204]each A, C, D, and E is independently a natural or non-natural amino acid;
[0205]each B is independently a natural or non-natural amino acid, amino acid analogue,
[0000]
[0000][—NH-L3-CO—], [—NH-L3-SO2—], or [—NH-L3-];
[0206]each R1and R2is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, unsubstituted or substituted with halo-;
[0207]each R3is independently hydrogen, alkyl, alkenyl, alkynyl, arylalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, cycloalkylalkyl, aryl, or heteroaryl, optionally substituted with R5;
[0208]each L is independently a macrocycle-forming linker of the formula
[0000]
[0209]each L1, L2and L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, heteroarylene, or [—R4—K—R4-]n, each being optionally substituted with R5;
[0210]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene;
[0211]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0212]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0213]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0214]each R7is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, optionally substituted with R5, or part of a cyclic structure with a D residue;
[0215]each R8is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, optionally substituted with R5, or part of a cyclic structure with an E residue;
[0216]each v and w is independently an integer from 1-1000;
[0217]u is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10;
[0218]each x, y and z is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; and
[0219]n is 1, 2, 3, 4, or 5.
[0220]In other embodiments, provided are peptidomimetic macrocycles comprising Formula (II) or (IIa):
[0000]
[0221]each A, C, D, and E is independently a natural or non-natural amino acid, and the terminal D and E independently optionally include a capping group;
[0222]each B is independently a natural or non-natural amino acid, amino acid analogue,
[0000]
[0000][—NH-L3-CO—], [—NH-L3-SO2—], or [—NH-L3-];
[0223]each R1and R2is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, unsubstituted or substituted with halo-; or at least one of R1and R2forms a macrocycle-forming linker U connected to the alpha position of one of said D or E amino acids;
[0224]each R3is independently hydrogen, alkyl, alkenyl, alkynyl, arylalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, cycloalkylalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5;
[0225]each L1, L2, and L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, cycloarylene, heterocycloarylene, or [—R4—K—R4-]n, each being optionally substituted with R5;
[0226]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene;
[0227]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0228]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0229]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0230]each R7is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5;
[0231]each v and w is independently an integer from 0-100;
[0232]u is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10;
[0233]each x, y and z is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10;
[0234]n is 1, 2, 3, 4, or 5; and
[0235]A, B, C, and E, taken together with the crosslinked amino acids connected by the macrocycle-forming linker -L1-L2-, form an amino acid sequence of the peptidomimetic macrocycle which is at least about 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 100% identical to a sequence of Table 1.
[0236]In some embodiments, a peptidomimetic macrocycle comprises Formula (IIIa) or (IIIb):
[0000]
[0237]each A, C, D and E is independently an amino acid, and the terminal D and E independently optionally include a capping group;
[0238]each B is independently an amino acid,
[0000]
[0000][—NH-L3-CO—], [—NH-L3-SO2—], or [—NH-L3-];
[0239]each R1′ and R2is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, unsubstituted or substituted with halo-; or R2forms a macrocycle-forming linker L′ connected to the alpha position of one of said E amino acids;
[0240]each R3is independently hydrogen, alkyl, alkenyl, alkynyl, arylalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, cycloalkylalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5;
[0241]each L and L′ is independently a macrocycle-forming linker of the formula -L1-L2-,
[0000]
[0242]each L1, L2and L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, cycloarylene, heterocycloarylene, or [—R4—K—R4-]n, each being optionally substituted with R5;
[0243]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene;
[0244]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0245]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0246]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0247]each R7or R7′ is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5, or part of a cyclic structure with a D residue;
[0248]each R8or R8′ is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5, or part of a cyclic structure with an E residue;
[0249]each R9is independently absent, hydrogen, alkyl, alkenyl, alkynyl, aryl, cycloalkyl, cycloalkenyl, heteroaryl, or heterocyclyl group, unsubstituted or optionally substituted with Raor Rb;
[0250]each Raand Rbis independently alkyl, OCH3, CF3, NH2, CH2NH2, F, Br, I,
[0000]
[0251]each v′ and w is independently an integer from 0-1000, for example 0-500, 0-200, 0-100, 0-50, 0-30, 0-20, or 0-10;
[0252]u is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, for example 1, 2, 3, 4, or 5; 1, 2, or 3; or 1 or 2;
[0253]each x, y, z, x′, y′ and z′ is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, for example the sum of x+y+z is 2, 3, 6 or 10, or the sum of x′+y′+z′ is 2, 3, 6, or 10;
[0254]n is 1, 2, 3, 4, or 5;
[0255]X is C═O, CHRc, or C═S;
[0256]Rcis alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl; and
[0257]A, B, C, and E, taken together with the crosslinked amino acids connected by the macrocycle-forming linker -L1-L2-, form an amino acid sequence of the peptidomimetic macrocycle which is at least about 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 96%, 97%, 98%, 99%, or 100% identical to a sequence of Table 1.
[0258]In some embodiments, the peptidomimetic macrocycle has the Formula:
[0000]
[0259]each R1′ or R2′ is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, unsubstituted or substituted with halo-; and
[0260]each v, w, v′ or w′ is independently an integer from 0-100.
[0261]In some embodiments, the notation “Hep” is used for a macrocycle of Formula Ma, which represents an N-terminal heptenoic capping group of the following formula:
[0000]
[0000]wherein AA1, AA2, AA3and AA4are amino acids.
[0262]In other embodiments, a C-terminal macrocycle of Formula IIIb forms the structure:
[0000]
[0263]In some embodiments, the peptidomimetic macrocycle has the Formula IV:
[0000]
[0264]each A, C, D, and E is independently an amino acid;
[0265]each B is independently an amino acid,
[0000]
[0000][—NH-L4-CO—], [—NH-L4-SO2—], or [—NH-L4-];
[0266]each R1and R2is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, unsubstituted or substituted with halo-; or at least one of R1and R2forms a macrocycle-forming linker L′ connected to the alpha position of one of said D or E amino acids;
[0267]each R3is independently hydrogen, alkyl, alkenyl, alkynyl, arylalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, cycloalkylalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5;
[0268]each L1, L2, L3and L4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, cycloarylene, heterocycloarylene or [—R4—K—R4]n, each being unsubstituted or substituted with R5;
[0269]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0270]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene;
[0271]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0272]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope or a therapeutic agent;
[0273]each R7is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5, or part of a cyclic structure with a D residue;
[0274]each R8is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl, optionally substituted with R5, or part of a cyclic structure with an E residue;
[0275]each v and w is independently an integer from 1-1000, for example 1-500, 1-200, 1-100, 1-50, 1-30, 1-20 or 1-10;
[0276]u is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, for example 1, 2, 3, 4, or 5; 1, 2, or 3; or 1 or 2;
[0277]each x, y and z is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, for example the sum of x+y+z is 2, 3, 6 or 10, for example sum of x+y+z is 2, 3 or 6; and
[0278]n is 1, 2, 3, 4, or 5.
[0279]In some embodiments, the peptidomimetic macrocycle has the Formula (V):
[0000]
[0280]each D and E is independently an amino acid residue;
[0281]R1and R2are independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, each being optionally substituted with halo-; or at least one of R1and R2forms a macrocycle-forming linker L′ connected to the alpha position of one of the D or E amino acid residues;
[0282]each L or L′ is independently a macrocycle-forming linker of the formula -L1-L2- or -L1-L2-L3-;
[0283]each L1, L2, and L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, heteroarylene, or [—R4—K—R4-]n, each being optionally substituted with R5;
[0284]each R3is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5;
[0285]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5;
[0286]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0287]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0288]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0289]R7is —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with a D residue;
[0290]R8is —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with an E residue;
[0291]each of Xaa1and Xaa2is independently an amino acid residue or absent;
[0292]Xaa3is Ala, Aib, Asp, Asn, Cys, Glu, Gln, His, Ile, Lys, Leu, Met, Arg, Ser, Thr, Val, Trp, Tyr, or an analogue of any of the foregoing;
[0293]v is an integer from 1-1000;
[0294]w is an integer from 0-1000; and
[0295]n is 1, 2, 3, 4, or 5.
[0296]In some embodiments, the peptidomimetic macrocycle of Formula (V) comprises two crosslinks, wherein a first crosslink is of a first pair of amino acid residues, and a second crosslink is of a second pair of amino acid residues. In some embodiments, the first pair of amino acid residues and the second pair of amino acid residues do not share a common amino acid residue. In some embodiments, the first pair of amino acid residues and the second pair of amino acid residues share one common amino acid residue. In some embodiments, one of Xaa1and Xaa2is His. In some embodiments, both of Xaa1and Xaa2are His. In some embodiments, one of Xaa1and Xaa2is Arg. In some embodiments, both of Xaa1and Xaa2are Arg. In some embodiments, one of Xaa1and Xaa2is absent. In some embodiments, both of Xaa1and Xaa2are absent.
[0297]In some embodiments, the peptidomimetic macrocycle comprises a helix. In some embodiments, the peptidomimetic macrocycle comprises an α-helix. In some embodiments, v is 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15. In some embodiments, v is 3, 4, 5, 6, 7, 8, 9, or 10. In some embodiments, v is 8. In some embodiments, w is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15. In some embodiments, w is 0, 1, 2, 3, 4, or 5. In some embodiments, w is 0, 1, 2, or 3. In some embodiments, wherein w is 0.
[0298]In some embodiments, each v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30. In some embodiments, w is an integer from 3-1000, for example 3-500, 3-200, 3-100, 3-50, 3-30, 3-20, or 3-10. In some embodiments, the sum of x+y+z is 3 or 6. In some embodiments, the sum of x+y+z is 3. In other embodiments, the sum of x+y+z is 6.
[0299]In some embodiments, w is 3, 4, 5, 6, 7, 8, 9, or 10, for example 3, 4, 5, or 6; 3, 4, 5, 6, 7, or 8; 6, 7, or 8; or 6, 7, 8, 9, or 10. In some embodiments, w is 3. In other embodiments, w is 6. In some embodiments, v is an integer from 1-1000, for example 1-500, 1-200, 1-100, 1-50, 1-30, 1-20, or 1-10. In some embodiments, v is 2.
[0300]In some embodiments, L is the formula -L1-L2-, and L1and L2are independently alkylene, alkenylene, or alkynylene. In some embodiments, wherein L is the formula -L1-L2-, and L1and L2are independently C3-C10alkylene or C3-C10alkenylene. In some embodiments, wherein L is the formula -L1-L2-, and L1and L2are independently C3-C6alkylene or C3-C6alkenylene. In some embodiments, L is
[0000]
[0000]In some embodiments, L is the formula -L1-L2-L3-, and L1and L3are independently alkylene, alkenylene, or alkynylene, and L2is arylene or heteroarylene. In some embodiments, L is the formula -L1-L2-L3-, and L1and L3are independently C3-C10alkylene, and L2is heteroarylene. In some embodiments, L is the formula -L1-L2-L3-, and L1and L3are independently C3-C6alkylene, and L2is heteroarylene.
[0301]In some embodiments, R1and R2are H. In some embodiments, R1and R2are independently alkyl. In some embodiments, R1and R2are methyl.
[0302]In some embodiments, the peptidomimetic macrocycle has the Formula (VI) (SEQ ID NO: 1785):
[0000]
[0303]each D and E is independently an amino acid residue;
[0304]R1and R2are independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, each being optionally substituted with halo-; or at least one of R1and R2forms a macrocycle-forming linker L′ connected to the alpha position of one of the D or E amino acid residues;
[0305]each L or L′ is independently a macrocycle-forming linker of the formula -L1-L2- or -L1-L2-L3-;
[0306]each L1, L2, and L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, heteroarylene, or [—R4—K—R4-]n, each being optionally substituted with R5;
[0307]each R3is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5;
[0308]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5;
[0309]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0310]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0311]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0312]R7is —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with a D residue;
[0313]R8is —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with an E residue;
[0314]each of Xaa1and Xaa2is independently an amino acid residue or absent;
[0315]v is an integer from 1-1000;
[0316]w is an integer from 0-1000; and
[0317]n is 1, 2, 3, 4, or 5.
[0318]In some embodiments, the peptidomimetic macrocycle of Formula (VI) comprises two crosslinks, wherein a first crosslink is of a first pair of amino acid residues, and a second crosslink is of a second pair of amino acid residues. In some embodiments, the first pair of amino acid residues and the second pair of amino acid residues do not share a common amino acid residue. In some embodiments, the first pair of amino acid residues and the second pair of amino acid residues share one common amino acid residue. In some embodiments, one of Xaa1and Xaa2is His. In some embodiments, both of Xaa1and Xaa2are His. In some embodiments, one of Xaa1and Xaa2is Arg. In some embodiments, both of Xaa1and Xaa2are Arg. In some embodiments, one of Xaa1and Xaa2is absent. In some embodiments, both of Xaa1and Xaa2are absent.
[0319]In some embodiments, the peptidomimetic macrocycle comprises a helix. In some embodiments, the peptidomimetic macrocycle comprises an α-helix. In some embodiments, v is 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15. In some embodiments, v is 3, 4, 5, 6, 7, 8, 9, or 10. In some embodiments, v is 8. In some embodiments, w is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15. In some embodiments, w is 0, 1, 2, 3, 4, or 5. In some embodiments, w is 0, 1, 2, or 3. In some embodiments, wherein w is 0.
[0320]In some embodiments, each v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, or 30. In some embodiments, w is an integer from 3-1000, for example 3-500, 3-200, 3-100, 3-50, 3-30, 3-20, or 3-10. In some embodiments, the sum of x+y+z is 3 or 6. In some embodiments, the sum of x+y+z is 3. In other embodiments, the sum of x+y+z is 6.
[0321]In some embodiments, w is 3, 4, 5, 6, 7, 8, 9, 10, for example 3, 4, 5, or 6; 3, 4, 5, 6, 7, or 8; 6, 7, or 8; or 6, 7, 8, 9, or 10. In some embodiments, w is 3. In other embodiments, w is 6. In some embodiments, v is an integer from 1-1000, for example 1-500, 1-200, 1-100, 1-50, 1-30, 1-20, or 1-10. In some embodiments, v is 2.
[0322]In some embodiments, L is the formula -L1-L2-, and L1and L2are independently alkylene, alkenylene, or alkynylene. In some embodiments, wherein L is the formula -L1-L2-, and L1and L2are independently C3-C10alkylene or C3-C10alkenylene. In some embodiments, wherein L is the formula -L1-L2-, and L1and L2are independently C3-C6alkylene or C3-C6alkenylene. In some embodiments, L is
[0000]
[0000]In some embodiments, L is the formula -L1-L2-L3-, and L1and L3are independently alkylene, alkenylene, or alkynylene, and L2is arylene or heteroarylene. In some embodiments, L is the formula -L1-L2-L3-, and L1and L3are independently C3-C10alkylene, and L2is heteroarylene. In some embodiments, L is the formula -L1-L2-L3-, and L1and L3are independently C3-C6alkylene, and L2is heteroarylene.
[0323]In some embodiments, R1and R2are H. In some embodiments, R1and R2are independently alkyl. In some embodiments, R1and R2are methyl.
[0324]In one example, at least one of R1and R2is alkyl, unsubstituted or substituted with halo-. In another example, both R1and R2are independently alkyl, unsubstituted or substituted with halo-. In some embodiments, at least one of R1and R2is methyl. In other embodiments, R1and R2are methyl.
[0325]In some embodiments of the invention, the sum of the sum of x+y+z is at least 3, or the sum of x′+y′+z′ is at least 3. In other embodiments of the invention, the sum of the sum of x+y+z is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 (for example 2, 3 or 6) or the sum of x′+y′+z′ is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10 (for example 2, 3 or 6).
[0326]Each occurrence of A, B, C, D or E in a macrocycle or macrocycle precursor of the invention is independently selected. For example, a sequence represented by the formula [A]x, when x is 3, encompasses embodiments where the amino acids are not identical, e.g. Gln-Asp-Ala as well as embodiments where the amino acids are identical, e.g. Gln-Gln-Gln. This applies for any value of x, y, or z in the indicated ranges. Similarly, when u is greater than 1, each compound of the invention may encompass peptidomimetic macrocycles which are the same or different. For example, a compound of the invention may comprise peptidomimetic macrocycles comprising different linker lengths or chemical compositions.
[0327]In some embodiments, the peptidomimetic macrocycle of the invention comprises a secondary structure which is an α-helix and R8is —H, allowing intrahelical hydrogen bonding. In some embodiments, at least one of A, B, C, D or E is an α,α-disubstituted amino acid. In one example, B is an α,α-disubstituted amino acid. For instance, at least one of A, B, C, D or E is 2-aminoisobutyric acid. In other embodiments, at least one of A, B, C, D or E is
[0000]
[0328]In other embodiments, the length of the macrocycle-forming linker L as measured from a first Cα to a second Cα is selected to stabilize a desired secondary peptide structure, such as an α-helix formed by residues of the peptidomimetic macrocycle including, but not necessarily limited to, those between the first Cα to a second Cα.
[0329]In one embodiment, the peptidomimetic macrocycle of Formula (I) is:
[0000]
[0000]wherein each R1and R2is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, unsubstituted or substituted with halo-.
[0330]In related embodiments, the peptidomimetic macrocycle comprises a structure of Formula (I) which is:
[0000]
[0331]In other embodiments, the peptidomimetic macrocycle of Formula (I) is a compound of any of the formulas shown below:
[0000]wherein “AA” represents any natural or non-natural amino acid side chain and “ ” is [D]v, [E]was defined above, and n is an integer between 0 and 20, 50, 100, 200, 300, 400 or 500. In some embodiments, the substituent “n” shown in the preceding paragraph is 0. In other embodiments, the substituent “n” shown in the preceding paragraph is less than 50, 40, 30, 20, 10, or 5.
” is [D]v, [E]was defined above, and n is an integer between 0 and 20, 50, 100, 200, 300, 400 or 500. In some embodiments, the substituent “n” shown in the preceding paragraph is 0. In other embodiments, the substituent “n” shown in the preceding paragraph is less than 50, 40, 30, 20, 10, or 5.
[0332]Exemplary embodiments of the macrocycle-forming linker L are shown below.
[0000]
[0333]In other embodiments, D or E in the compound of Formula I are further modified in order to facilitate cellular uptake. In some embodiments, lipidating or PEGylating a peptidomimetic macrocycle facilitates cellular uptake, increases bioavailability, increases blood circulation, alters pharmacokinetics, decreases immunogenicity or decreases the needed frequency of administration.
[0334]In other embodiments, at least one of [D] and [E] in the compound of Formula I represents a moiety comprising an additional macrocycle-forming linker such that the peptidomimetic macrocycle comprises at least two macrocycle-forming linkers. In a specific embodiment, a peptidomimetic macrocycle comprises two macrocycle-forming linkers.
[0335]In the peptidomimetic macrocycles of the invention, any of the macrocycle-forming linkers described herein may be used in any combination with any of the sequences shown in Tables 1-2 and also with any of the R— substituents indicated herein.
[0336]In some embodiments, the peptidomimetic macrocycle comprises at least one α-helix motif. For example, A, B or C in the compound of Formula I include one or more α-helices. As a general matter, α-helices include between 3 and 4 amino acid residues per turn. In some embodiments, the α-helix of the peptidomimetic macrocycle includes 1 to 5 turns and, therefore, 3 to 20 amino acid residues. In specific embodiments, the α-helix includes 1 turn, 2 turns, 3 turns, 4 turns, or 5 turns. In some embodiments, the macrocycle-forming linker stabilizes an α-helix motif included within the peptidomimetic macrocycle. Thus, in some embodiments, the length of the macrocycle-forming linker L from a first Cα to a second Cα is selected to increase the stability of an α-helix. In some embodiments, the macrocycle-forming linker spans from 1 turn to 5 turns of the α-helix. In some embodiments, the macrocycle-forming linker spans approximately 1 turn, 2 turns, 3 turns, 4 turns, or 5 turns of the α-helix. In some embodiments, the length of the macrocycle-forming linker is approximately 5 Å to 9 Å per turn of the α-helix, or approximately 6 Å to 8 Å per turn of the α-helix. Where the macrocycle-forming linker spans approximately 1 turn of an α-helix, the length is equal to approximately 5 carbon-carbon bonds to 13 carbon-carbon bonds, approximately 7 carbon-carbon bonds to 11 carbon-carbon bonds, or approximately 9 carbon-carbon bonds. Where the macrocycle-forming linker spans approximately 2 turns of an α-helix, the length is equal to approximately 8 carbon-carbon bonds to 16 carbon-carbon bonds, approximately 10 carbon-carbon bonds to 14 carbon-carbon bonds, or approximately 12 carbon-carbon bonds. Where the macrocycle-forming linker spans approximately 3 turns of an α-helix, the length is equal to approximately 14 carbon-carbon bonds to 22 carbon-carbon bonds, approximately 16 carbon-carbon bonds to 20 carbon-carbon bonds, or approximately 18 carbon-carbon bonds. Where the macrocycle-forming linker spans approximately 4 turns of an α-helix, the length is equal to approximately 20 carbon-carbon bonds to 28 carbon-carbon bonds, approximately 22 carbon-carbon bonds to 26 carbon-carbon bonds, or approximately 24 carbon-carbon bonds. Where the macrocycle-forming linker spans approximately 5 turns of an α-helix, the length is equal to approximately 26 carbon-carbon bonds to 34 carbon-carbon bonds, approximately 28 carbon-carbon bonds to 32 carbon-carbon bonds, or approximately 30 carbon-carbon bonds. Where the macrocycle-forming linker spans approximately 1 turn of an α-helix, the linkage contains approximately 4 atoms to 12 atoms, approximately 6 atoms to 10 atoms, or approximately 8 atoms. Where the macrocycle-forming linker spans approximately 2 turns of the α-helix, the linkage contains approximately 7 atoms to 15 atoms, approximately 9 atoms to 13 atoms, or approximately 11 atoms. Where the macrocycle-forming linker spans approximately 3 turns of the α-helix, the linkage contains approximately 13 atoms to 21 atoms, approximately 15 atoms to 19 atoms, or approximately 17 atoms. Where the macrocycle-forming linker spans approximately 4 turns of the α-helix, the linkage contains approximately 19 atoms to 27 atoms, approximately 21 atoms to 25 atoms, or approximately 23 atoms. Where the macrocycle-forming linker spans approximately 5 turns of the α-helix, the linkage contains approximately 25 atoms to 33 atoms, approximately 27 atoms to 31 atoms, or approximately 29 atoms. Where the macrocycle-forming linker spans approximately 1 turn of the α-helix, the resulting macrocycle forms a ring containing approximately 17 members to 25 members, approximately 19 members to 23 members, or approximately 21 members. Where the macrocycle-forming linker spans approximately 2 turns of the α-helix, the resulting macrocycle forms a ring containing approximately 29 members to 37 members, approximately 31 members to 35 members, or approximately 33 members. Where the macrocycle-forming linker spans approximately 3 turns of the α-helix, the resulting macrocycle forms a ring containing approximately 44 members to 52 members, approximately 46 members to 50 members, or approximately 48 members. Where the macrocycle-forming linker spans approximately 4 turns of the α-helix, the resulting macrocycle forms a ring containing approximately 59 members to 67 members, approximately 61 members to 65 members, or approximately 63 members. Where the macrocycle-forming linker spans approximately 5 turns of the α-helix, the resulting macrocycle forms a ring containing approximately 74 members to 82 members, approximately 76 members to 80 members, or approximately 78 members.
[0337]In some embodiments, L is a macrocycle-forming linker of the formula:
[0000]
[0338]Exemplary embodiments of such macrocycle-forming linkers L are shown below.
[0339]In some embodiments, the peptidomimetic macrocycle comprises an amino acid sequence of formula:
X1-X2-X3-X4-X5-X6-X7-X8-X9-X10-X11-X12-X13-X14-X15-X16-X17-X18-X19-X20-X21
[0341]In some embodiments, X1 is Ile, Arg, Ala, Lys, Pro, Leu, Asp, Glu, His, Ser, Gln, Phe, an analogue thereof, or absent.
[0342]In some embodiments, X2 is Trp, Arg, Ala, Asn, Phe, Pro, Leu, Ser, Lys, Tyr, His, Cou, Cou2, Cou4, Cou7, an analogue thereof, a crosslinked amino acid, or absent.
[0343]In some embodiments, X3 is Ile, Ala, Leu, Phe, Tyr, Val, Asp, Trp, Pro, Gln, Chg, Ac5c, Ac6c, Tba, Bip, Cha, Adm, hCha, an analogue thereof, or absent.
[0344]In some embodiments, X4 is Ala, Gln, Asp, Val, Gly, Ser, Leu, Phe, Cha, A4, an analogue, thereof, a crosslinked amino acid, or absent.
[0345]In some embodiments, X5 is Gln, Ala, Leu, Phe, Tyr, Gly, Ile, Val, Arg, Glu, Pro, Asp, MO, MO2, an analogue thereof, a crosslinked amino acid, or absent.
[0346]In some embodiments, X6 is Glu, Gln, His, Ala, Ser, Arg, Ile, Leu, Thr, Phe, Val, Tyr, Gly, Nle, St, an analogue thereof, or absent.
[0347]In some embodiments, X7 is Ala, Leu, Phe, Ile, 2Nal, 1Nal, 3cf, Chg, Cha, Adm, hCha, Igl, Bip, an analogue thereof, or absent.
[0348]In some embodiments, X8 is Arg, Ala, Asp, Glu, Thr, His, Gln, Gly, Asn, Phe, Cit, St, an analogue thereof, a crosslinked amino acid, or absent.
[0349]In some embodiments, X9 is Arg, Ala, Asp, Lys, Asn, Gly, Ser, Gln, Cys, Nle, St, an analogue thereof, or a crosslinked amino acid.
[0350]In some embodiments, X10 is Ile, Val, Ala, Asp, Asn, Phe, Tba, hL, hhL, Nle, Chg, Cha, an analogue thereof, or a crosslinked amino acid.
[0351]In some embodiments, X11 is Gly, Val, Ala, Leu, Ile, Asp, Glu, Cha, Aib, Abu, an analogue thereof, or a crosslinked amino acid.
[0352]In some embodiments, X12 is Asp, Ala, Asn, Gly, Arg, Glu, Lys, Leu, Nle, an analogue thereof, or a crosslinked amino acid.
[0353]In some embodiments, X13 is Ala, Glu, Gln, Leu, Lys, Asp, Tyr, Ile, Ser, Cys, St, Sta5, Aib, Nle, an analogue thereof, or a crosslinked amino acid.
[0354]In some embodiments, X14 is Phe, Ala, Leu, Val, Tyr, Glu, His, Ile, Nle, 1Nal, 2Nal, Chg, Cha, BiP, an analogue thereof, or a crosslinked amino acid.
[0355]In some embodiments, X15 is Asn, Gln, Ser, His, Glu, Asp, Ala, Leu, Ile, St, Nle, Aib, an analogue thereof, a crosslinked amino acid, or absent.
[0356]In some embodiments, X16 is Ala, Glu, Asp, Arg, Lys, Phe, Gly, Gln, Aib, Cha, St, an analogue thereof, a crosslinked amino acid, or absent.
[0357]In some embodiments, X17 is Phe, Tyr, Ala, Leu, Asn, Ser, Gln, Arg, His, Thr, Cou2, Cou3, Cou7, Dpr, Amf, Damf, Amye, an analogue thereof, a crosslinked amino acid, or absent.
[0358]In some embodiments, X18 is Tyr, Ala, Ile, Phe, His, Arg, Lys, Trp, Orn, Amf, Amye, Cha, 2Nal, an analogue thereof, or absent.
[0359]In some embodiments, X19 is Ala, Lys, Arg, His, Ser, Gln, Glu, Asp, Thr, Aib, Cha, an analogue thereof, a crosslinked amino acid, or absent.
[0360]In some embodiments, X20 is Arg, His, Ala, Thr, Lys, Amr, an analogue thereof, a crosslinked amino acid, or absent.
[0361]In some embodiments, X21 is Arg, His, Ala, Amr, an analogue thereof, or absent.
[0362]In some embodiments, the peptidomimetic macrocycle comprises a helix.
[0363]In some embodiments, the peptidomimetic macrocycle comprises an α-helix.
[0364]In some embodiments, the peptidomimetic macrocycle comprises an α,α-disubstituted amino acid.
[0365]In some embodiments, each amino acid connected by the macrocycle-forming linker is an α,α-disubstituted amino acid.
Warhead-Containing Peptidomimetic Macrocycles
[0366]The binding sites of the target proteins can be populated with amino acids that are capable of covalent modification with suitable reactive ligands. In some embodiments, the peptidomimetic macrocycles of the invention contain at least one warhead that can covalently modify a target protein. Non-limiting examples of a target protein include Bfl-1 and Bcl-2 family proteins.
[0367]In some embodiments, amino acids that are capable of covalent modification with suitable reactive ligands can be located near or in the binding regions of the peptidomimetic macrocycles of the invention. Amino acids capable of covalent modification are amino acids with heteroatoms in the side chain, such as threonine, cysteine, histidine, serine, tyrosine, and lysine. Amino acids such as lysine are unreactive and do not react in vivo. In some embodiments, a hydrogen bond donor amino acid in proximity to a lysine moiety can enhance the nucleophilicity of the lysine nitrogen by lowering the pKa, and make lysine reactive toward an electrophilic warhead.
[0368]Amino acids with hydrogen donor capability include arginine, threonine, serine, histidine, tyrosine, and lysine. In some embodiments, hydrogen bond donation by a side chain or a main chain amide can enhance the electrophilicity of a warhead. The compounds of the invention can incorporate an amino acid warhead to be proximal to a lysine or cysteine amino acid of a target protein to facilitate the formation of a covalent bond and irreversibly inhibit the target protein.
[0369]In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention are designed to be proximal to a Lys or Cys amino acid of the target protein to form a covalent bond for the irreversible inhibition of the target protein. In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention act as irreversible inhibitors that covalently bind to their target proteins.
[0370]In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention can permanently eliminate existing drug target activity, which can return when the target protein is newly synthesized. In some embodiments, the therapeutic plasma concentration of a compound can irreversibly suppress the activity of a target protein. In some embodiments, the plasma levels of a target protein can decline while the target protein remains inactivated. In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention can lower the minimal blood plasma concentration required for therapeutic activity. In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention can minimize dosing requirements. In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention can eliminate the requirement for long plasma-half lives. In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention can reduce toxicity resulting from any nonspecific off-target interactions that can occur at high or prolonged blood plasma levels.
[0371]In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention can inactivate target proteins that have resistance mutations. In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention can have enhanced potency, which may lower the dose of inhibitor required to silence the target protein.
[0372]In some embodiments, the peptidomimetic macrocycles of the invention comprise at least one warhead. In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention comprise an amino acid sequence that is about 60%, about 70%, about 80%, about 90%, about 95%, and about 99% identical to an amino acid sequence identified as binding to the binding site of a target protein.
[0373]In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention are of the formula:
[0000]
[0374]each A, C, D, and E is independently a natural or non-natural amino acid;
[0375]each B is independently a natural or non-natural amino acid, amino acid analogue,
[0000]
[0000][—NH-L3-CO—], [—NH-L3-SO2—], or [—NH-L3-];
[0376]each L is independently a macrocycle-forming linker;
[0377]each L′ is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5, or a bond, or together with R1and the atom to which both R1and L′ are bound forms a ring;
[0378]each L″ is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5, or a bond, or together with R2and the atom to which both R2and L″ are bound forms a ring;
[0379]each R1is independently-H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, each being optionally substituted with halo-, or together with L′ and the atom to which both R1and L′ are bound forms a ring;
[0380]each R2is independently-H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, each being optionally substituted with halo-, or together with L″ and the atom to which both R2and L″ are bound forms a ring;
[0381]each R3is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, cycloalkylalkyl, aryl, or heteroaryl, each being optionally substituted with R5;
[0382]each L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, heteroarylene, or [—R4—K—R4-]n, each being optionally substituted with R5;
[0383]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene;
[0384]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0385]each n is independently 1, 2, 3, 4, or 5;
[0386]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0387]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0388]each R7is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with a D residue;
[0389]each R8is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with an E residue;
[0390]each v and w is independently an integer from 1-1000;
[0391]u is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; and
[0392]each x, y and z is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, or
[0000]a pharmaceutically-acceptable salt thereof, wherein the peptidomimetic macrocycle comprises an amino acid with an electron accepting group susceptible to attack by a nucleophile.
[0393]In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention are of the formula:
[0000]
[0394]each A, C, D, E, and F is independently a natural or non-natural amino acid;
[0395]each B is independently a natural or non-natural amino acid, amino acid analogue,
[0000]
[0000][—NH-L3-CO—], [—NH-L3-SO2—], or [—NH-L3-];
[0396]each WH is an amino acid with an electron accepting group susceptible to attack by a nucleophile;
[0397]each L is independently a macrocycle-forming linker;
[0398]each L′ is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5, or a bond, or together with R1and the atom to which both R1and L′ are bound forms a ring;
[0399]each L″ is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5, or a bond, or together with R2and the atom to which both R2and L″ are bound forms a ring;
[0400]each R1is independently-H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, each being optionally substituted with halo-, or together with L′ and the atom to which both R1and L′ are bound forms a ring;
[0401]each R2is independently-H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, each being optionally substituted with halo-, or together with L″ and the atom to which both R2and L″ are bound forms a ring;
[0402]each R3is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, cycloalkylalkyl, aryl, or heteroaryl, each being optionally substituted with R5;
[0403]each L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, heteroarylene, or [—R4—K—R4-]n, each being optionally substituted with R5;
[0404]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene;
[0405]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0406]each n is independently 1, 2, 3, 4, or 5;
[0407]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0408]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0409]each R7is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with a D residue;
[0410]each R8is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with an E residue;
[0411]each v and w is independently an integer from 1-1000;
[0412]t is 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10;
[0413]u is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; and
[0414]each x, y and z is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, or
[0000]a pharmaceutically-acceptable salt thereof.
[0415]In some embodiments, t is 0, 1, or 2. In some embodiments, t is 0. In some embodiments, u is 1 or 2. In some embodiments, t is 0, and u is 1.
[0416]In some embodiments, the warhead (WH)-containing peptidomimetic macrocycles of the invention are of the formula:
[0000]
[0417]In some embodiments, the warhead-containing peptidomimetic macrocycles are of the formula:
[0000]
[0418]In some embodiments, the warhead-containing peptidomimetic macrocycles of the invention comprise an amino acid of the formula:
[0000]
[0000]In some embodiments, the warhead of the amino acids are of the formula:
[0000]
[0000]wherein:
- X is alkylene, CH, CH2, NRα, O, or S, wherein Rαis —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl;
- Rαis H, CN, or C(O)CH3;
- Rbis H, methyl, ethyl, allyl, propyl, isopropyl, butyl, or isobutyl;
- each Rc, Rd, and Reis independently —H, C1-C4saturated or unsaturated, straight or branched, hydrocarbon chain, or an electron-withdrawing group, and in some embodiments, at least one of Rc, Rd, and Reis an electron withdrawing group;
- Rfis halogen, a C2alkynyl or alkenyl side chain optionally substituted with oxo, halogen, NO2, or CN; and
- n′ iso, 1, 2, 3, 4, or 5.
[0425]In some embodiments, Rdand Reare each independently —H, methyl, ethyl, allyl, propyl, isopropyl, butyl, or isobutyl. In some embodiments, Rfis —CH═CH2or —C≡CH.
[0426]In some embodiments, the warhead-containing peptidomimetic macrocycles of the formula comprise an amino acid with the side chain:
[0000]
[0427]In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NOs: 1-1625 and one Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NOs: 1-500 and one Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NOs: 1-10 and one Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NOs: 1500-1625 and one Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NOs: 1575-1625 and one Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NOs: 1620-1625 and one Michael acceptor.
[0428]In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NOs: 1-1625 and
[0000]
[0000]as a Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NOs: 1575-1625 and
[0000]
[0000]as a Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NOs: 1-50 or 1620-1625 and
[0000]
[0000]as a Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NO 2 with
[0000]
[0000]as a Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NO 15 with
[0000]
[0000]as a Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NO 1620 with
[0000]
[0000]as a Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NO 1621 with
[0000]
[0000]as a Michael acceptor. In some embodiments, the peptidomimetic macrocycles of the invention comprise SEQ ID NO 1625 with
[0000]
[0000]as a Michael acceptor.
[0429]Non-limiting examples of warhead-containing peptidomimetic macrocycles include:
[WH]IAQELR$IGD$FNAYYARR-NH2(SEQ ID NO: 1626) and
[WH]IAQALR$r8hLGDAFN$YF4FA-NH2(SEQ ID NO: 1627).
Preparation of Peptidomimetic Macrocycles
[0430]Peptidomimetic macrocycles of the invention may be prepared by any of a variety of methods known in the art. For example, any of the residues indicated by “X”, “Z” or “XX” in Tables for 2 may be substituted with a residue capable of forming a crosslinker with a second residue in the same molecule or a precursor of such a residue.
[0431]Various methods to effect formation of peptidomimetic macrocycles are known in the art. For example, the preparation of peptidomimetic macrocycles of Formula I is described in Schafmeister et al., J. Am. Chem. Soc. 122:5891-5892 (2000); Schafmeister & Verdin, J. Am. Chem. Soc. 122:5891 (2005); Walensky et al., Science 305:1466-1470 (2004); U.S. Pat. No. 7,192,713 and PCT application WO 2008/121767. The α,α-disubstituted amino acids and amino acid precursors disclosed in the cited references may be employed in synthesis of the peptidomimetic macrocycle precursor polypeptides. For example, the “55-olefin amino acid” is (S)-α-(2′-pentenyl) alanine and the “R8 olefin amino acid” is (R)-α-(2′-octenyl) alanine. Following incorporation of such amino acids into precursor polypeptides, the terminal olefins are reacted with a metathesis catalyst, leading to the formation of the peptidomimetic macrocycle. In various embodiments, the following amino acids may be employed in the synthesis of the peptidomimetic macrocycle:
[0000]
[0432]In some embodiments, x+y+z is 3, and A, B and C are independently natural or non-natural amino acids. In other embodiments, x+y+z is 6, and A, B and C are independently natural or non-natural amino acids.
[0433]In some embodiments, the contacting step is performed in a solvent selected from the group consisting of protic solvent, aqueous solvent, organic solvent, and mixtures thereof. For example, the solvent may be chosen from the group consisting of H2O, THF, THF/H2O, tBuOH/H2O, DMF, DIPEA, CH3CN or CH2Cl2, ClCH2CH2Cl or a mixture thereof. The solvent may be a solvent which favors helix formation.
[0434]Alternative but equivalent protecting groups, leaving groups or reagents are substituted, and certain of the synthetic steps are performed in alternative sequences or orders to produce the desired compounds. Synthetic chemistry transformations and protecting group methodologies (protection and deprotection) useful in synthesizing the compounds described herein include, for example, those such as described in Larock, Comprehensive Organic Transformations, VCH Publishers (1989); Greene and Wuts, Protective Groups in Organic Synthesis, 2d. Ed., John Wiley and Sons (1991); Fieser and Fieser, Fieser and Fieser's Reagents for Organic Synthesis, John Wiley and Sons (1994); and Paquette, ed., Encyclopedia of Reagents for Organic Synthesis, John Wiley and Sons (1995), and subsequent editions thereof.
[0435]The peptidomimetic macrocycles disclosed herein are made, for example, by chemical synthesis methods, such as described in Fields et al., Chapter 3 in Synthetic Peptides: A User's Guide, ed. Grant, W. H. Freeman & Co., New York, N.Y., 1992, p. 77. Hence, for example, peptides are synthesized using the automated Merrifield techniques of solid phase synthesis with the amine protected by either tBoc or Fmoc chemistry using side chain protected amino acids on, for example, an automated peptide synthesizer (e.g., Applied Biosystems (Foster City, Calif.), Model 430A, 431, or 433).
[0436]One manner of producing the peptidomimetic precursors and peptidomimetic macrocycles described herein uses solid phase peptide synthesis (SPPS). The C-terminal amino acid is attached to a cross-linked polystyrene resin via an acid labile bond with a linker molecule. This resin is insoluble in the solvents used for synthesis, making it relatively simple and fast to wash away excess reagents and by-products. The N-terminus is protected with the Fmoc group, which is stable in acid, but removable by base. Side chain functional groups are protected as necessary with base stable, acid labile groups.
[0437]Longer peptidomimetic precursors are produced, for example, by conjoining individual synthetic peptides using native chemical ligation. Alternatively, the longer synthetic peptides are biosynthesized by well-known recombinant DNA and protein expression techniques. Such techniques are provided in well-known standard manuals with detailed protocols. To construct a gene encoding a peptidomimetic precursor of this invention, the amino acid sequence is reverse translated to obtain a nucleic acid sequence encoding the amino acid sequence, preferably with codons that are optimum for the organism in which the gene is to be expressed. Next, a synthetic gene is made, typically by synthesizing oligonucleotides which encode the peptide and any regulatory elements, if necessary. The synthetic gene is inserted in a suitable cloning vector and transfected into a host cell. The peptide is then expressed under suitable conditions appropriate for the selected expression system and host. The peptide is purified and characterized by standard methods.
[0438]The peptidomimetic precursors are made, for example, in a high-throughput, combinatorial fashion using, for example, a high-throughput polychannel combinatorial synthesizer (e.g., Thuramed TETRAS multichannel peptide synthesizer from CreoSalus, Louisville, Ky. or Model Apex 396 multichannel peptide synthesizer from AAPPTEC, Inc., Louisville, Ky.).
[0439]In some embodiments, the peptidomimetic macrocycles of the invention comprise triazole macrocycle-forming linkers. For example, the synthesis of such peptidomimetic macrocycles involves a multi-step process that features the synthesis of a peptidomimetic precursor containing an azide moiety and an alkyne moiety; followed by contacting the peptidomimetic precursor with a macrocyclization reagent to generate a triazole-linked peptidomimetic macrocycle. Such a process is described, for example, in U.S. application Ser. No. 12/037,041, filed on Feb. 25, 2008. Macrocycles or macrocycle precursors are synthesized, for example, by solution phase or solid-phase methods, and can contain both naturally-occurring and non-naturally-occurring amino acids. See, for example, Hunt, “The Non-Protein Amino Acids” in Chemistry and Biochemistry of the Amino Acids, edited by G. C. Barrett, Chapman and Hall, 1985.
[0440]In some embodiments, an azide is linked to the α-carbon of a residue and an alkyne is attached to the α-carbon of another residue. In some embodiments, the azide moieties are azido-analogues of amino acids L-lysine, D-lysine, alpha-methyl-L-lysine, alpha-methyl-D-lysine, L-ornithine, D-ornithine, alpha-methyl-L-ornithine or alpha-methyl-D-ornithine. In another embodiment, the alkyne moiety is L-propargylglycine. In yet other embodiments, the alkyne moiety is an amino acid selected from the group consisting of L-propargylglycine, D-propargylglycine, (S)-2-amino-2-methyl-4-pentynoic acid, (R)-2-amino-2-methyl-4-pentynoic acid, (S)-2-amino-2-methyl-5-hexynoic acid, (R)-2-amino-2-methyl-5-hexynoic acid, (S)-2-amino-2-methyl-6-heptynoic acid, (R)-2-amino-2-methyl-6-heptynoic acid, (S)-2-amino-2-methyl-7-octynoic acid, (R)-2-amino-2-methyl-7-octynoic acid, (S)-2-amino-2-methyl-8-nonynoic acid and (R)-2-amino-2-methyl-8-nonynoic acid.
[0441]The following synthetic schemes are provided solely to illustrate the present invention and are not intended to limit the scope of the invention, as described herein. To simplify the drawings, the illustrative schemes depict azido amino acid analogues ε-azido-α-methyl-L-lysine and ε-azido-α-methyl-D-lysine, and alkyne amino acid analogues L-propargylglycine, (S)-2-amino-2-methyl-4-pentynoic acid, and (S)-2-amino-2-methyl-6-heptynoic acid. Thus, in the following synthetic schemes, each R1, R2, R7and R8is —H; each L1is —(CH2)4—; and each L2is —(CH2)—. However, as noted throughout the detailed description above, many other amino acid analogues can be employed in which R1, R2, R7, R8, L1and L2can be independently selected from the various structures disclosed herein.
[0442]Synthetic Scheme 1 describes the preparation of several compounds of the invention. Ni(II) complexes of Schiff bases derived from the chiral auxiliary (S)-2-[N—(N′-benzylprolyl)amino]benzophenone (BPB) and amino acids such as glycine or alanine are prepared as described in Belokon et al. (1998), Tetrahedron Asymm. 9:4249-4252. The resulting complexes are subsequently reacted with alkylating reagents comprising an azido or alkynyl moiety to yield enantiomerically enriched compounds of the invention. If desired, the resulting compounds can be protected for use in peptide synthesis.
[0000]
[0443]In the general method for the synthesis of peptidomimetic macrocycles shown in Synthetic Scheme 2, the peptidomimetic precursor contains an azide moiety and an alkyne moiety and is synthesized by solution-phase or solid-phase peptide synthesis (SPPS) using the commercially available amino acid N-α-Fmoc-L-propargylglycine and the N-α-Fmoc-protected forms of the amino acids (S)-2-amino-2-methyl-4-pentynoic acid, (S)-2-amino-6-heptynoic acid, (S)-2-amino-2-methyl-6-heptynoic acid, N-methyl-ε-azido-L-lysine, and N-methyl-ε-azido-D-lysine. The peptidomimetic precursor is then deprotected and cleaved from the solid-phase resin by standard conditions (e.g., strong acid such as 95% TFA). The peptidomimetic precursor is reacted as a crude mixture or is purified prior to reaction with a macrocyclization reagent such as a Cu(I) in organic or aqueous solutions (Rostovtsev et al. (2002), Angew. Chem. Int. Ed. 41:2596-2599; Tornoe et al. (2002), J. Org. Chem. 67:3057-3064; Deiters et al. (2003), J. Am. Chem. Soc. 125:11782-11783; Punna et al. (2005), Angew. Chem. Int. Ed. 44:2215-2220). In one embodiment, the triazole forming reaction is performed under conditions that favor α-helix formation. In one embodiment, the macrocyclization step is performed in a solvent chosen from the group consisting of H2O, THF, CH3CN, DMF, DIPEA, tBuOH or a mixture thereof. In another embodiment, the macrocyclization step is performed in DMF. In some embodiments, the macrocyclization step is performed in a buffered aqueous or partially aqueous solvent.
[0000]
[0444]In the general method for the synthesis of peptidomimetic macrocycles shown in Synthetic Scheme 3, the peptidomimetic precursor contains an azide moiety and an alkyne moiety and is synthesized by solid-phase peptide synthesis (SPPS) using the commercially available amino acid N-α-Fmoc-L-propargylglycine and the N-α-Fmoc-protected forms of the amino acids (S)-2-amino-2-methyl-4-pentynoic acid, (S)-2-amino-6-heptynoic acid, (S)-2-amino-2-methyl-6-heptynoic acid, N-methyl-ε-azido-L-lysine, and N-methyl-ε-azido-D-lysine. The peptidomimetic precursor is reacted with a macrocyclization reagent such as a Cu(I) reagent on the resin as a crude mixture (Rostovtsev et al. (2002), Angew. Chem. Int. Ed. 41:2596-2599; Tornoe et al. (2002), J. Org. Chem. 67:3057-3064; Deiters et al. (2003), J. Am. Chem. Soc. 125:11782-11783; Punna et al. (2005), Angew. Chem. Int. Ed. 44:2215-2220). The resultant triazole-containing peptidomimetic macrocycle is then deprotected and cleaved from the solid-phase resin by standard conditions (e.g., strong acid such as 95% TFA). In some embodiments, the macrocyclization step is performed in a solvent chosen from the group consisting of CH2Cl2, ClCH2CH2Cl, DMF, THF, NMP, DIPEA, 2,6-lutidine, pyridine, DMSO, H2O or a mixture thereof. In some embodiments, the macrocyclization step is performed in a buffered aqueous or partially aqueous solvent.
[0000]
[0445]In the general method for the synthesis of peptidomimetic macrocycles shown in Synthetic Scheme 4, the peptidomimetic precursor contains an azide moiety and an alkyne moiety and is synthesized by solution-phase or solid-phase peptide synthesis (SPPS) using the commercially available amino acid N-α-Fmoc-L-propargylglycine and the N-α-Fmoc-protected forms of the amino acids (S)-2-amino-2-methyl-4-pentynoic acid, (S)-2-amino-6-heptynoic acid, (S)-2-amino-2-methyl-6-heptynoic acid, N-methyl-ε-azido-L-lysine, and N-methyl-ε-azido-D-lysine. The peptidomimetic precursor is then deprotected and cleaved from the solid-phase resin by standard conditions (e.g., strong acid such as 95% TFA). The peptidomimetic precursor is reacted as a crude mixture or is purified prior to reaction with a macrocyclization reagent such as a Ru(II) reagents, for example Cp*RuCl(PPh3)2or [Cp*RuCl]4(Rasmussen et al. (2007), Org. Lett. 9:5337-5339; Zhang et al. (2005), J. Am. Chem. Soc. 127:15998-15999). In some embodiments, the macrocyclization step is performed in a solvent chosen from the group consisting of DMF, CH3CN and THF.
[0000]
[0446]In the general method for the synthesis of peptidomimetic macrocycles shown in Synthetic Scheme 5, the peptidomimetic precursor contains an azide moiety and an alkyne moiety and is synthesized by solid-phase peptide synthesis (SPPS) using the commercially available amino acid N-α-Fmoc-L-propargylglycine and the N-α-Fmoc-protected forms of the amino acids (S)-2-amino-2-methyl-4-pentynoic acid, (S)-2-amino-6-heptynoic acid, (S)-2-amino-2-methyl-6-heptynoic acid, N-methyl-ε-azido-L-lysine, and N-methyl-ε-azido-D-lysine. The peptidomimetic precursor is reacted with a macrocyclization reagent such as a Ru(II) reagent on the resin as a crude mixture. For example, the reagent can be Cp*RuCl(PPh3)2or [Cp*RuCl]4(Rasmussen et al. (2007), Org. Lett. 9:5337-5339; Zhang et al. (2005), J. Am. Chem. Soc. 127:15998-15999). In some embodiments, the macrocyclization step is performed in a solvent chosen from the group consisting of CH2Cl2, ClCH2CH2Cl, CH3CN, DMF, and THF.
[0447]In some embodiments, a peptidomimetic macrocycle of Formula I comprises a halogen group substitution on a triazole moiety, for example an iodo substitution. Such peptidomimetic macrocycles may be prepared from a precursor having the partial structure and using the cross-linking methods taught herein. Crosslinkers of any length, as described herein, may be prepared comprising such substitutions. In one embodiment, the peptidomimetic macrocycle is prepared according to the scheme shown below. The reaction is performed, for example, in the presence of CuI and an amine ligand such as TEA or TTTA. See, e.g., Hein et al. Angew. Chem., Int. Ed. 2009, 48, 8018-8021.
[0000]
[0448]In other embodiments, an iodo-substituted triazole is generated according to the scheme shown below. For example, the second step in the reaction scheme below is performed using, for example, CuI and N-bromosuccinimide (NBS) in the presence of THF (see, e.g. Zhang et al., J. Org. Chem. 2008, 73, 3630-3633). In other embodiments, the second step in the reaction scheme shown below is performed, for example, using CuI and an iodinating agent such as ICl (see, e.g. Wu et al., Synthesis 2005, 1314-1318.)
[0000]
[0449]In some embodiments, an iodo-substituted triazole moiety is used in a cross-coupling reaction, such as a Suzuki or Sonogashira coupling, to afford a peptidomimetic macrocycle comprising a substituted crosslinker. Sonogashira couplings using an alkyne as shown below may be performed, for example, in the presence of a palladium catalyst such as Pd(PPh3)2Cl2, CuI, and in the presence of a base such as triethylamine. Suzuki couplings using an arylboronic or substituted alkenyl boronic acid as shown below may be performed, for example, in the presence of a catalyst such as Pd(PPh3)4, and in the presence of a base such as K2CO3.
[0000]
[0450]Any suitable triazole substituent groups which reacts with the iodo-substituted triazole can be used in Suzuki couplings described herein. Example triazole substituents for use in Suzuki couplings are shown below:
[0000]
[0000]wherein “Cyc” is a suitable aryl, cycloalkyl, cycloalkenyl, heteroaryl, or heterocyclyl group, unsubstituted or optionally substituted with an Raor Rbgroup as described below.
[0451]In some embodiments, the substituent is:
[0000]
[0452]Any suitable substituent group which reacts with the iodo-substituted triazole can be used in Sonogashira couplings described herein. Example triazole substituents for use in Sonogashira couplings are shown below:
[0000]
[0000]wherein “Cyc” is a suitable aryl, cycloalkyl, cycloalkenyl, heteroaryl, or heterocyclyl group, unsubstituted or optionally substituted with an Raor Rbgroup as described below.
[0453]In some embodiments, the triazole substituent is:
[0000]
[0454]In some embodiments, the Cyc group shown above is further substituted by at least one Raor Rbsubstituent. In some embodiments, at least one of Raand Rbis independently:
[0000]
[0455]In other embodiments, the triazole substituent is
[0000]
[0000]and at least one of Raand Rbis alkyl (including hydrogen, methyl, or ethyl), or:
[0000]
[0000]The present invention contemplates the use of non-naturally-occurring amino acids and
[0456]The present invention contemplates the use of non-naturally-occurring amino acids and amino acid analogues in the synthesis of the peptidomimetic macrocycles described herein. Any amino acid or amino acid analogue amenable to the synthetic methods employed for the synthesis of stable triazole containing peptidomimetic macrocycles can be used in the present invention. For example, L-propargylglycine is contemplated as a useful amino acid in the present invention. However, other alkyne-containing amino acids that contain a different amino acid side chain are also useful in the invention. For example, L-propargylglycine contains one methylene unit between the α-carbon of the amino acid and the alkyne of the amino acid side chain. The invention also contemplates the use of amino acids with multiple methylene units between the α-carbon and the alkyne. Also, the azido-analogues of amino acids L-lysine, D-lysine, alpha-methyl-L-lysine, and alpha-methyl-D-lysine are contemplated as useful amino acids in the present invention. However, other terminal azide amino acids that contain a different amino acid side chain are also useful in the invention. For example, the azido-analogue of L-lysine contains four methylene units between the α-carbon of the amino acid and the terminal azide of the amino acid side chain. The invention also contemplates the use of amino acids with fewer than or greater than four methylene units between the α-carbon and the terminal azide. Table 2 shows some amino acids useful in the preparation of peptidomimetic macrocycles disclosed herein.
[0457]In some embodiments the amino acids and amino acid analogues are of the D-configuration. In other embodiments they are of the L-configuration. In some embodiments, some of the amino acids and amino acid analogues contained in the peptidomimetic are of the D-configuration while some of the amino acids and amino acid analogues are of the L-configuration. In some embodiments the amino acid analogues are α,α-disubstituted, such as α-methyl-L-propargylglycine, α-methyl-D-propargylglycine, ε-azido-alpha-methyl-L-lysine, and ε-azido-alpha-methyl-D-lysine. In some embodiments the amino acid analogues are N-alkylated, e.g., N-methyl-L-propargylglycine, N-methyl-D-propargylglycine, N-methyl-ε-azido-L-lysine, and N-methyl-ε-azido-D-lysine.
[0458]In some embodiments, the —NH moiety of the amino acid is protected using a protecting group, including without limitation -Fmoc and -Boc. In other embodiments, the amino acid is not protected prior to synthesis of the peptidomimetic macrocycle.
[0459]In some embodiments, the —NH moiety of the amino acid is protected using a protecting group, including without limitation -Fmoc and -Boc. In other embodiments, the amino acid is not protected prior to synthesis of the peptidomimetic macrocycle.
[0460]The preparation of macrocycles of Formula IV is described, for example, in U.S. application Ser. No. 11/957,325, filed on Dec. 17, 2007 and herein incorporated by reference. Synthetic Schemes 6-9 describe the preparation of such compounds of Formula IV. To simplify the drawings, the illustrative schemes depict amino acid analogues derived from L- or D-cysteine, in which L1and L3are both —(CH2)—. However, as noted throughout the detailed description above, many other amino acid analogues can be employed in which L1and L3can be independently selected from the various structures disclosed herein. The symbols “[AA]m”, “[AA]n”, “[AA]o” represent a sequence of amide bond-linked moieties such as natural or unnatural amino acids. As described previously, each occurrence of “AA” is independent of any other occurrence of “AA”, and a formula such as “[AA]m” encompasses, for example, sequences of non-identical amino acids as well as sequences of identical amino acids.
[0000]

[0461]In Scheme 6, the peptidomimetic precursor contains two —SH moieties and is synthesized by solid-phase peptide synthesis (SPPS) using commercially available N-α-Fmoc amino acids such as N-α-Fmoc-S-trityl-L-cysteine or N-α-Fmoc-S-trityl-D-cysteine. Alpha-methylated versions of D-cysteine or L-cysteine are generated by known methods (Seebach et al. (1996), Angew. Chem. Int. Ed. Engl. 35:2708-2748, and references therein) and then converted to the appropriately protected N-α-Fmoc-S-trityl monomers by known methods (“Bioorganic Chemistry: Peptides and Proteins”, Oxford University Press, New York: 1998, the entire contents of which are incorporated herein by reference). The precursor peptidomimetic is then deprotected and cleaved from the solid-phase resin by standard conditions (e.g., strong acid such as 95% TFA). The precursor peptidomimetic is reacted as a crude mixture or is purified prior to reaction with X-L2-Y in organic or aqueous solutions. In some embodiments the alkylation reaction is performed under dilute conditions (i.e. 0.15 mmol/L) to favor macrocyclization and to avoid polymerization. In some embodiments, the alkylation reaction is performed in organic solutions such as liquid NH3(Mosberg et al. (1985), J. Am. Chem. Soc. 107:2986-2987; Szewczuk et al. (1992), Int. J. Peptide Protein Res. 40:233-242), NH3/MeOH, or NH3/DMF (Or et al. (1991), J. Org. Chem. 56:3146-3149). In other embodiments, the alkylation is performed in an aqueous solution such as 6M guanidinium HCL, pH 8 (Brunel et al. (2005), Chem. Commun. (20):2552-2554). In other embodiments, the solvent used for the alkylation reaction is DMF or dichloroethane.
[0000]

[0462]In Scheme 7, the precursor peptidomimetic contains two or more —SH moieties, of which two are specially protected to allow their selective deprotection and subsequent alkylation for macrocycle formation. The precursor peptidomimetic is synthesized by solid-phase peptide synthesis (SPPS) using commercially available N-α-Fmoc amino acids such as N-α-Fmoc-S-p-methoxytrityl-L-cysteine or N-α-Fmoc-S-p-methoxytrityl-D-cysteine. Alpha-methylated versions of D-cysteine or L-cysteine are generated by known methods (Seebach et al. (1996), Angew. Chem. Int. Ed. Engl. 35:2708-2748, and references therein) and then converted to the appropriately protected N-α-Fmoc-S-p-methoxytrityl monomers by known methods (Bioorganic Chemistry: Peptides and Proteins, Oxford University Press, New York: 1998, the entire contents of which are incorporated herein by reference). The Mmt protecting groups of the peptidomimetic precursor are then selectively cleaved by standard conditions (e.g., mild acid such as 1% TFA in DCM). The precursor peptidomimetic is then reacted on the resin with X-L2-Y in an organic solution. For example, the reaction takes place in the presence of a hindered base such as diisopropylethylamine. In some embodiments, the alkylation reaction is performed in organic solutions such as liquid NH3(Mosberg et al. (1985), J. Am. Chem. Soc. 107:2986-2987; Szewczuk et al. (1992), Int. J. Peptide Protein Res. 40:233-242), NH3/MeOH or NH3/DMF (Or et al. (1991), J. Org. Chem. 56:3146-3149). In other embodiments, the alkylation reaction is performed in DMF or dichloroethane. The peptidomimetic macrocycle is then deprotected and cleaved from the solid-phase resin by standard conditions (e.g., strong acid such as 95% TFA).
[0000]
[0463]In Scheme 8, the peptidomimetic precursor contains two or more —SH moieties, of which two are specially protected to allow their selective deprotection and subsequent alkylation for macrocycle formation. The peptidomimetic precursor is synthesized by solid-phase peptide synthesis (SPPS) using commercially available N-α-Fmoc amino acids such as N-α-Fmoc-S-p-methoxytrityl-L-cysteine, N-α-Fmoc-S-p-methoxytrityl-D-cysteine, N-α-Fmoc-S—S-t-butyl-L-cysteine, and N-α-Fmoc-S—S-t-butyl-D-cysteine. Alpha-methylated versions of D-cysteine or L-cysteine are generated by known methods (Seebach et al. (1996), Angew. Chem. Int. Ed. Engl. 35:2708-2748, and references therein) and then converted to the appropriately protected N-α-Fmoc-S-p-methoxytrityl or N-α-Fmoc-S—S-t-butyl monomers by known methods (Bioorganic Chemistry: Peptides and Proteins, Oxford University Press, New York: 1998, the entire contents of which are incorporated herein by reference). The S—S-tButyl protecting group of the peptidomimetic precursor is selectively cleaved by known conditions (e.g., 20% 2-mercaptoethanol in DMF, reference: Galande et al. (2005), J. Comb. Chem. 7:174-177). The precursor peptidomimetic is then reacted on the resin with a molar excess of X-L2-Y in an organic solution. For example, the reaction takes place in the presence of a hindered base such as diisopropylethylamine. The Mmt protecting group of the peptidomimetic precursor is then selectively cleaved by standard conditions (e.g., mild acid such as 1% TFA in DCM). The peptidomimetic precursor is then cyclized on the resin by treatment with a hindered base in organic solutions. In some embodiments, the alkylation reaction is performed in organic solutions such as NH3/MeOH or NH3/DMF (Or et al. (1991), J. Org. Chem. 56:3146-3149). The peptidomimetic macrocycle is then deprotected and cleaved from the solid-phase resin by standard conditions (e.g., strong acid such as 95% TFA).
[0000]
[0464]In Scheme 9, the peptidomimetic precursor contains two L-cysteine moieties. The peptidomimetic precursor is synthesized by known biological expression systems in living cells or by known in vitro, cell-free, expression methods. The precursor peptidomimetic is reacted as a crude mixture or is purified prior to reaction with X-L2-Y in organic or aqueous solutions. In some embodiments the alkylation reaction is performed under dilute conditions (i.e. 0.15 mmol/L) to favor macrocyclization and to avoid polymerization. In some embodiments, the alkylation reaction is performed in organic solutions such as liquid NH3(Mosberg et al. (1985), J. Am. Chem. Soc. 107:2986-2987; Szewczuk et al. (1992), Int. J. Peptide Protein Res. 40:233-242), NH3/MeOH, or NH3/DMF (Or et al. (1991), J. Org. Chem. 56:3146-3149). In other embodiments, the alkylation is performed in an aqueous solution such as 6M guanidinium HCL, pH 8 (Brunel et al. (2005), Chem. Commun. (20):2552-2554). In other embodiments, the alkylation is performed in DMF or dichloroethane. In another embodiment, the alkylation is performed in non-denaturing aqueous solutions, and in yet another embodiment the alkylation is performed under conditions that favor α-helical structure formation. In yet another embodiment, the alkylation is performed under conditions that favor the binding of the precursor peptidomimetic to another protein, so as to induce the formation of the bound α-helical conformation during the alkylation.
[0465]Various embodiments for X and Y are envisioned which are suitable for reacting with thiol groups. In general, each X or Y is independently be selected from the general category shown in Table 3. For example, X and Y are halides such as —Cl, —Br or —I. Any of the macrocycle-forming linkers described herein may be used in any combination with any of the sequences shown and also with any of the R-substituents indicated herein.
[0000] |
| Examples of Reactive Groups Capable of Reacting |
| with Thiol Groups and Resulting Linkages |
| | Resulting Covalent |
| X or Y | Linkage |
| |
| acrylamide | Thioether |
| halide (e.g. alkyl or aryl halide) | Thioether |
| sulfonate | Thioether |
| aziridine | Thioether |
| epoxide | Thioether |
| haloacetamide | Thioether |
| maleimide | Thioether |
| sulfonate ester | Thioether |
| |
[0466]The present invention contemplates the use of both naturally occurring and non-naturally-occurring amino acids and amino acid analogues in the synthesis of the peptidomimetic macrocycles of Formula IV. Any amino acid or amino acid analogue amenable to the synthetic methods employed for the synthesis of stable bis-sulfhydryl containing peptidomimetic macrocycles can be used in the present invention. For example, cysteine is contemplated as a useful amino acid in the present invention. However, sulfur containing amino acids other than cysteine that contain a different amino acid side chain are also useful. For example, cysteine contains one methylene unit between the α-carbon of the amino acid and the terminal-SH of the amino acid side chain. The invention also contemplates the use of amino acids with multiple methylene units between the α-carbon and the terminal —SH. Non-limiting examples include α-methyl-L-homocysteine and α-methyl-D-homocysteine. In some embodiments the amino acids and amino acid analogues are of the D-configuration. In other embodiments they are of the L-configuration. In some embodiments, some of the amino acids and amino acid analogues contained in the peptidomimetic are of the D-configuration while some of the amino acids and amino acid analogues are of the L-configuration. In some embodiments the amino acid analogues are α,α-disubstituted, such as α-methyl-L-cysteine and α-methyl-D-cysteine.
[0467]The invention includes macrocycles in which macrocycle-forming linkers are used to link two or more —SH moieties in the peptidomimetic precursors to form the peptidomimetic macrocycles disclosed herein. As described above, the macrocycle-forming linkers impart conformational rigidity, increased metabolic stability or increased cell penetrability. Furthermore, in some embodiments, the macrocycle-forming linkages stabilize the α-helical secondary structure of the peptidomimetic macrocycles. The macrocycle-forming linkers are of the formula X-L2-Y, wherein both X and Y are the same or different moieties, as defined above. Both X and Y have the chemical characteristics that allow one macrocycle-forming linker-L2- to bis alkylate the bis-sulfhydryl containing peptidomimetic precursor. As defined above, the linker-L2-includes alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, cycloarylene, or heterocycloarylene, or —R4—K—R4—, all of which can be optionally substituted with an R5group, as defined above. Furthermore, one to three carbon atoms within the macrocycle-forming linkers-L2-, other than the carbons attached to the —SH of the sulfhydryl containing amino acid, are optionally substituted with a heteroatom such as N, S or O.
[0468]The L2component of the macrocycle-forming linker X-L2-Y may be varied in length depending on, among other things, the distance between the positions of the two amino acid analogues used to form the peptidomimetic macrocycle. Furthermore, as the lengths of L1or L3components of the macrocycle-forming linker are varied, the length of L2can also be varied in order to create a linker of appropriate overall length for forming a stable peptidomimetic macrocycle. For example, if the amino acid analogues used are varied by adding an additional methylene unit to each of L1and L3, the length of L2are decreased in length by the equivalent of approximately two methylene units to compensate for the increased lengths of L1and L3.
[0469]In some embodiments, L2is an alkylene group of the formula —(CH2)n—, where n is 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15. For example, n is 1, 2, 3, 4, 5, 6, 7, 8, 9 or 10. In other embodiments, L2is an alkenylene group. In still other embodiments, L2is an aryl group.
[0470]Table 4 shows additional embodiments of X-L2-Y groups.
[0471]Additional methods of forming peptidomimetic macrocycles which are envisioned as suitable to perform the present invention include those disclosed by Mustapa, M. Firouz Mohd et al., J. Org. Chem (2003), 68, pp. 8193-8198; Yang, Bin et al. Bioorg Med. Chem. Lett. (2004), 14, pp. 1403-1406; U.S. Pat. No. 5,364,851; U.S. Pat. No. 5,446,128; U.S. Pat. No. 5,824,483; U.S. Pat. No. 6,713,280; and U.S. Pat. No. 7,202,332. In such embodiments, amino acid precursors are used containing an additional substituent R— at the alpha position. Such amino acids are incorporated into the macrocycle precursor at the desired positions, which may be at the positions where the crosslinker is substituted or, alternatively, elsewhere in the sequence of the macrocycle precursor. Cyclization of the precursor is then performed according to the indicated method.
[0472]For example, a peptidomimetic macrocycle of Formula (II) is prepared as indicated:
[0000]
[0000]wherein each AA1, AA2, AA3 is independently an amino acid side chain.
[0473]In other embodiments, a peptidomimetic macrocycle of Formula (II) is prepared as indicated:
[0000]
[0000]wherein each AA1, AA2, AA3 is independently an amino acid side chain.
[0474]In some embodiments, a peptidomimetic macrocycle is obtained in more than one isomer, for example due to the configuration of a double bond within the structure of the crosslinker (E vs Z). Such isomers can or cannot be separable by conventional chromatographic methods. In some embodiments, one isomer has improved biological properties relative to the other isomer. In one embodiment, an E crosslinker olefin isomer of a peptidomimetic macrocycle has better solubility, better target affinity, better in vivo or in vitro efficacy, higher helicity, or improved cell permeability relative to its Z counterpart. In another embodiment, a Z crosslinker olefin isomer of a peptidomimetic macrocycle has better solubility, better target affinity, better in vivo or in vitro efficacy, higher helicity, or improved cell permeability relative to its E counterpart.
[0475]A compound described herein can be at least 1% pure, at least 2% pure, at least 3% pure, at least 4% pure, at least 5% pure, at least 6% pure, at least 7% pure, at least 8% pure, at least 9% pure, at least 10% pure, at least 11% pure, at least 12% pure, at least 13% pure, at least 14% pure, at least 15% pure, at least 16% pure, at least 17% pure, at least 18% pure, at least 19% pure, at least 20% pure, at least 21% pure, at least 22% pure, at least 23% pure, at least 24% pure, at least 25% pure, at least 26% pure, at least 27% pure, at least 28% pure, at least 29% pure, at least 30% pure, at least 31% pure, at least 32% pure, at least 33% pure, at least 34% pure, at least 35% pure, at least 36% pure, at least 37% pure, at least 38% pure, at least 39% pure, at least 40% pure, at least 41% pure, at least 42% pure, at least 43% pure, at least 44% pure, at least 45% pure, at least 46% pure, at least 47% pure, at least 48% pure, at least 49% pure, at least 50% pure, at least 51% pure, at least 52% pure, at least 53% pure, at least 54% pure, at least 55% pure, at least 56% pure, at least 57% pure, at least 58% pure, at least 59% pure, at least 60% pure, at least 61% pure, at least 62% pure, at least 63% pure, at least 64% pure, at least 65% pure, at least 66% pure, at least 67% pure, at least 68% pure, at least 69% pure, at least 70% pure, at least 71% pure, at least 72% pure, at least 73% pure, at least 74% pure, at least 75% pure, at least 76% pure, at least 77% pure, at least 78% pure, at least 79% pure, at least 80% pure, at least 81% pure, at least 82% pure, at least 83% pure, at least 84% pure, at least 85% pure, at least 86% pure, at least 87% pure, at least 88% pure, at least 89% pure, at least 90% pure, at least 91% pure, at least 92% pure, at least 93% pure, at least 94% pure, at least 95% pure, at least 96% pure, at least 97% pure, at least 98% pure, at least 99% pure, at least 99.1% pure, at least 99.2% pure, at least 99.3% pure, at least 99.4% pure, at least 99.5% pure, at least 99.6% pure, at least 99.7% pure, at least 99.8% pure, or at least 99.9% pure on a chemical, optical, isomeric, enantiomeric, or diastereomeric basis. Purity can be assessed, for example, by HPLC, MS, LC/MS, melting point, or NMR.
Assays
[0476]The properties of the peptidomimetic macrocycles of the invention are assayed, for example, by using the methods described below. In some embodiments, a peptidomimetic macrocycle of the invention has improved biological properties relative to a corresponding polypeptide lacking the substituents described herein.
[0477]In some embodiments, a peptidomimetic macrocycle disclosed herein selectively binds BFL-1, or a BCL-2 family protein, selectively over another protein that has a BH3 domain. In some embodiments, the selectivity is a ratio of about 2 to about 1, about 3 to about 1, about 4 to about 1, about 5 to about 1, about 6 to about 1, about 7 to about 1, about 8 to about 1, about 9 to about 1, about 10 to about 1, about 20 to about 1, about 30 to about 1, about 40 to about 1, about 50 to about 1, about 60 to about 1, about 70 to about 1, about 80 to about 1, about 90 to about 1, about 100 to about 1, about 200 to about 1, about 300 to about 1, about 400 to about 1, about 500 to about 1, about 600 to about 1, about 700 to about 1, about 800 to about 1, about 900 to about 1, or about 1000 to about 1.
[0478]In some embodiments, a peptidomimetic macrocycle disclosed herein non-selectively binds additional types of proteins that have a BH3 domain. In some embodiments, the non-selectivity is at least about 2 types of proteins, at least about 3 types of proteins, at least about 4 types of proteins, at least about 5 types of proteins, at least about 6 types of proteins, at least about 7 types of proteins, at least about 8 types of proteins, at least about 9 types of proteins, at least about 10 types of proteins, at least about 11 types of protein, at least about 12 types of proteins, at least about 13 types of proteins, at least about 14 types of proteins, at least about 15 types of proteins, at least about 16 types of proteins, at least about 17 types of proteins, at least about 18 types of proteins, at least about 19 types of proteins, or at least about 20 types of proteins. In some embodiments, the non-selectivity is from about 2 types of protein to about 3 types of protein, from about 3 types of protein to about 4 types of protein, from about 4 types of protein to about 5 types of protein, from about 5 types of protein to about 6 types of protein, from about 6 types of protein to about 7 types of protein, from about 7 types of protein to about 8 types of protein, from about 8 types of protein to about 9 types of protein, from about 9 types of protein to about 10 types of protein, from about 10 types of protein to about 11 types of protein, from about 11 types of protein to about 12 types of protein, from about 12 types of protein to about 13 types of protein, from about 13 types of protein to about 14 types of protein, from about 14 types of protein to about 15 types of protein, from about 15 types of protein to about 16 types of protein, from about 16 types of protein to about 17 types of protein, from about 17 types of protein to about 18 types of protein, from about 18 types of protein to about 19 types of protein, or from about 19 types of protein to about 20 types of protein.
Assay to Determine α-Helicity.
[0479]In solution, the secondary structure of polypeptides with α-helical domains will reach a dynamic equilibrium between random coil structures and α-helical structures, often expressed as a “percent helicity”. Thus, for example, alpha-helical domains are predominantly random coils in solution, with α-helical content usually under 25%. Peptidomimetic macrocycles with optimized linkers, on the other hand, possess, for example, an alpha-helicity that is at least two-fold greater than that of a corresponding uncrosslinked polypeptide. In some embodiments, macrocycles of the invention will possess an alpha-helicity of greater than 50%. To assay the helicity of peptidomimetic macrocycles of the invention, the compounds are dissolved in an aqueous solution (e.g. 50 mM potassium phosphate solution at pH 7, or distilled H2O, to concentrations of 25-50 μM). Circular dichroism (CD) spectra are obtained on a spectropolarimeter (e.g., Jasco J-710) using standard measurement parameters (e.g. temperature, 20° C.; wavelength, 190-260 nm; step resolution, 0.5 nm; speed, 20 nm/sec; accumulations, 10; response, 1 sec; bandwidth, 1 nm; path length, 0.1 cm). The α-helical content of each peptide is calculated by dividing the mean residue ellipticity (e.g. [Φ]222obs) by the reported value for a model helical decapeptide (Yang et al. (1986), Methods Enzymol. 130:208)).
Assay to Determine Melting Temperature (Tm).
[0480]A peptidomimetic macrocycle of the invention comprising a secondary structure such as an α-helix exhibits, for example, a higher melting temperature than a corresponding uncrosslinked polypeptide. Typically peptidomimetic macrocycles of the invention exhibit Tm of >60° C. representing a highly stable structure in aqueous solutions. To assay the effect of macrocycle formation on melting temperature, peptidomimetic macrocycles or unmodified peptides are dissolved in distilled H2O (e.g. at a final concentration of 50 μM) and the Tm is determined by measuring the change in ellipticity over a temperature range (e.g. 4 to 95° C.) on a spectropolarimeter (e.g., Jasco J-710) using standard parameters (e.g. wavelength 222 nm; step resolution, 0.5 nm; speed, 20 nm/sec; accumulations, 10; response, 1 sec; bandwidth, 1 nm; temperature increase rate: 1° C./min; path length, 0.1 cm).
Protease Resistance Assay.
[0481]The amide bond of the peptide backbone is susceptible to hydrolysis by proteases, thereby rendering peptidic compounds vulnerable to rapid degradation in vivo. Peptide helix formation, however, typically buries the amide backbone and therefore may shield it from proteolytic cleavage. The peptidomimetic macrocycles of the present invention may be subjected to in vitro trypsin proteolysis to assess for any change in degradation rate compared to a corresponding uncrosslinked polypeptide. For example, the peptidomimetic macrocycle and a corresponding uncrosslinked polypeptide are incubated with trypsin agarose and the reactions quenched at various time points by centrifugation and subsequent HPLC injection to quantitate the residual substrate by ultraviolet absorption at 280 nm. Briefly, the peptidomimetic macrocycle and peptidomimetic precursor (5 mcg) are incubated with trypsin agarose (Pierce) (S/E ˜125) for 0, 10, 20, 90, and 180 minutes. Reactions are quenched by tabletop centrifugation at high speed; remaining substrate in the isolated supernatant is quantified by HPLC-based peak detection at 280 nm. The proteolytic reaction displays first order kinetics and the rate constant, k, is determined from a plot of 1n[S] versus time (k=−1×slope).
Ex Vivo Stability Assay.
[0482]Peptidomimetic macrocycles with optimized linkers possess, for example, an ex vivo half-life that is at least two-fold greater than that of a corresponding uncrosslinked polypeptide, and possess an ex vivo half-life of 12 hours or more. For ex vivo serum stability studies, a variety of assays may be used. For example, a peptidomimetic macrocycle and a corresponding uncrosslinked polypeptide (2 mcg) are incubated with fresh mouse, rat or human serum (2 mL) at 37° C. for 0, 1, 2, 4, 8, and 24 hours. To determine the level of intact compound, the following procedure may be used: The samples are extracted by transferring 100 μl of sera to 2 ml centrifuge tubes followed by the addition of 10 μL of 50% formic acid and 500 μL acetonitrile and centrifugation at 14,000 RPM for 10 min at 4±2° C. The supernatants are then transferred to fresh 2 ml tubes and evaporated on Turbovap under N2<10 psi, 37° C. The samples are reconstituted in 100 μL of 50:50 acetonitrile:water and submitted to LC-MS/MS analysis.
In Vitro Binding Assays.
[0483]To assess the binding and affinity of peptidomimetic macrocycles and peptidomimetic precursors to acceptor proteins, a fluorescence polarization assay (FPA) is used, for example. The FPA technique measures the molecular orientation and mobility using polarized light and fluorescent tracer. When excited with polarized light, fluorescent tracers (e.g., FITC) attached to molecules with high apparent molecular weights (e.g. FITC-labeled peptides bound to a large protein) emit higher levels of polarized fluorescence due to their slower rates of rotation as compared to fluorescent tracers attached to smaller molecules (e.g. FITC-labeled peptides that are free in solution).
[0484]For example, fluoresceinated peptidomimetic macrocycles (25 nM) are incubated with the acceptor protein (25-1000 nM) in binding buffer (140 mM NaCl, 50 mM Tris-HCL, pH 7.4) for 30 minutes at room temperature. Binding activity is measured, for example, by fluorescence polarization on a luminescence spectrophotometer (e.g. Perkin-Elmer LS50B). Kdvalues may be determined by nonlinear regression analysis using, for example, Graphpad Prism software (GraphPad Software, Inc., San Diego, Calif.). A peptidomimetic macrocycle of the invention shows, in some instances, similar or lower Kd than a corresponding uncrosslinked polypeptide.
In Vitro Displacement Assays to Characterize Antagonists of Peptide-Protein Interactions.
[0485]To assess the binding and affinity of compounds that antagonize the interaction between a peptide and an acceptor protein, a fluorescence polarization assay (FPA) utilizing a fluoresceinated peptidomimetic macrocycle derived from a peptidomimetic precursor sequence is used, for example. The FPA technique measures the molecular orientation and mobility using polarized light and fluorescent tracer. When excited with polarized light, fluorescent tracers (e.g., FITC) attached to molecules with high apparent molecular weights (e.g. FITC-labeled peptides bound to a large protein) emit higher levels of polarized fluorescence due to their slower rates of rotation as compared to fluorescent tracers attached to smaller molecules (e.g. FITC-labeled peptides that are free in solution). A compound that antagonizes the interaction between the fluoresceinated peptidomimetic macrocycle and an acceptor protein will be detected in a competitive binding FPA experiment.
[0486]For example, putative antagonist compounds (1 nM to 1 mM) and a fluoresceinated peptidomimetic macrocycle (25 nM) are incubated with the acceptor protein (50 nM) in binding buffer (140 mM NaCl, 50 mM Tris-HCL, pH 7.4) for 30 minutes at room temperature. Antagonist binding activity is measured, for example, by fluorescence polarization on a luminescence spectrophotometer (e.g. Perkin-Elmer LS50B). Kd values may be determined by nonlinear regression analysis using, for example, Graphpad Prism software (GraphPad Software, Inc., San Diego, Calif.).
[0487]Any class of molecule, such as small organic molecules, peptides, oligonucleotides or proteins can be examined as putative antagonists in this assay.
Assay for Protein-Ligand Binding by Affinity Selection-Mass Spectrometry.
[0488]To assess the binding and affinity of test compounds for proteins, an affinity-selection mass spectrometry assay is used, for example. Protein-ligand binding experiments are conducted according to the following representative procedure outlined for a system-wide control experiment using 1 μM peptidomimetic macrocycle plus 5 μM target protein. A 1 μL DMSO aliquot of a 40 μM stock solution of peptidomimetic macrocycle is dissolved in 19 μL of PBS (Phosphate-buffered saline: 50 mM, pH 7.5 Phosphate buffer containing 150 mM NaCl). The resulting solution is mixed by repeated pipetting and clarified by centrifugation at 10 000 g for 10 min. To a 4 μL aliquot of the resulting supernatant is added 4 μL of 10 μM target protein in PBS. Each 8.0 μL experimental sample thus contains 40 pmol (1.5 μg) of protein at 5.0 μM concentration in PBS plus 1 μM peptidomimetic macrocycle and 2.5% DMSO. Duplicate samples thus prepared for each concentration point are incubated for 60 min at room temperature, and then chilled to 4° C. prior to size-exclusion chromatography-LC-MS analysis of 5.0 μL injections. Samples containing a target protein, protein-ligand complexes, and unbound compounds are injected onto an SEC column, where the complexes are separated from non-binding component by a rapid SEC step. The SEC column eluate is monitored using UV detectors to confirm that the early-eluting protein fraction, which elutes in the void volume of the SEC column, is well resolved from unbound components that are retained on the column. After the peak containing the protein and protein-ligand complexes elutes from the primary UV detector, it enters a sample loop where it is excised from the flow stream of the SEC stage and transferred directly to the LC-MS via a valving mechanism. The (M+3H)3+ ion of the peptidomimetic macrocycle is observed by ESI-MS at the expected m/z, confirming the detection of the protein-ligand complex.
Assay for Protein-Ligand KdTitration Experiments.
[0489]To assess the binding and affinity of test compounds for proteins, a protein-ligand Kd titration experiment is performed. Protein-ligand Kdtitrations experiments are conducted as follows: 2 μL DMSO aliquots of a serially diluted stock solution of titrant peptidomimetic macrocycle (5, 2.5, . . . , 0.098 mM) are prepared then dissolved in 38 μL of PBS. The resulting solutions are mixed by repeated pipetting and clarified by centrifugation at 10 000 g for 10 min. To 4.0 μL aliquots of the resulting supernatants is added 4.0 μL of 10 μM target protein in PBS. Each 8.0 μL experimental sample thus contains 40 pmol (1.5 μg) of protein at 5.0 μM concentration in PBS, varying concentrations (125, 62.5, . . . , 0.24 μM) of the titrant peptide, and 2.5% DMSO. Duplicate samples thus prepared for each concentration point are incubated at room temperature for 30 min, then chilled to 4° C. prior to SEC-LC-MS analysis of 2.0 μL injections. The (M+H)1+, (M+2H)2+, (M+3H)3+, or (M+Na)1+ ion is observed by ESI-MS; extracted ion chromatograms are quantified, then fit to equations to derive the binding affinity Kdas described in “A General Technique to Rank Protein-Ligand Binding Affinities and Determine Allosteric vs. Direct Binding Site Competition in Compound Mixtures.” Annis, D. A.; Nazef, N.; Chuang, C. C.; Scott, M. P.; Nash, H. M. J. Am. Chem. Soc. 2004, 126, 15495-15503; also in “ALIS: An Affinity Selection-Mass Spectrometry System for the Discovery and Characterization of Protein-Ligand Interactions” D. A. Annis, C.-C. Chuang, and N. Nazef. In Mass Spectrometry in Medicinal Chemistry. Edited by Wanner K, Höfner G: Wiley-VCH; 2007:121-184. Mannhold R, Kubinyi H, Folkers G (Series Editors): Methods and Principles in Medicinal Chemistry.
Assay for Competitive Binding Experiments by Affinity Selection-Mass Spectrometry.
[0490]To determine the ability of test compounds to bind competitively to proteins, an affinity selection mass spectrometry assay is performed, for example. A mixture of ligands at 40 μM per component is prepared by combining 2 μL aliquots of 400 μM stocks of each of the three compounds with 14 μL of DMSO. Then, 1 μL aliquots of this 40 μM per component mixture are combined with 1 μL DMSO aliquots of a serially diluted stock solution of titrant peptidomimetic macrocycle (10, 5, 2.5, . . . , 0.078 mM). These 2 μL samples are dissolved in 38 μL of PBS. The resulting solutions were mixed by repeated pipetting and clarified by centrifugation at 10 000 g for 10 min. To 4.0 μL aliquots of the resulting supernatants is added 4.0 μL of 10 μM target protein in PBS. Each 8.0 μL experimental sample thus contains 40 pmol (1.5 μg) of protein at 5.0 μM concentration in PBS plus 0.5 μM ligand, 2.5% DMSO, and varying concentrations (125, 62.5, . . . , 0.98 μM) of the titrant peptidomimetic macrocycle. Duplicate samples thus prepared for each concentration point are incubated at room temperature for 60 min, then chilled to 4° C. prior to SEC-LC-MS analysis of 2.0 μL injections. Additional details on these and other methods are provided in “A General Technique to Rank Protein-Ligand Binding Affinities and Determine Allosteric vs. Direct Binding Site Competition in Compound Mixtures.” Annis, D. A.; Nazef, N.; Chuang, C. C.; Scott, M. P.; Nash, H. M. J. Am. Chem. Soc. 2004, 126, 15495-15503; also in “ALIS: An Affinity Selection-Mass Spectrometry System for the Discovery and Characterization of Protein-Ligand Interactions” D. A. Annis, C.-C. Chuang, and N. Nazef. In Mass Spectrometry in Medicinal Chemistry. Edited by Wanner K, Höfner G: Wiley-VCH; 2007:121-184. Mannhold R, Kubinyi H, Folkers G (Series Editors): Methods and Principles in Medicinal Chemistry.
Binding Assays in Intact Cells.
[0491]It is possible to measure binding of peptides or peptidomimetic macrocycles to their natural acceptors in intact cells by immunoprecipitation experiments. For example, intact cells are incubated with fluoresceinated (FITC-labeled) compounds for 4 hrs in the absence of serum, followed by serum replacement and further incubation that ranges from 4-18 hrs. Cells are then pelleted and incubated in lysis buffer (50 mM Tris [pH 7.6], 150 mM NaCl, 1% CHAPS and protease inhibitor cocktail) for 10 minutes at 4° C. Extracts are centrifuged at 14,000 rpm for 15 minutes and supernatants collected and incubated with 10 μl goat anti-FITC antibody for 2 hrs, rotating at 4° C. followed by further 2 hrs incubation at 4° C. with protein A/G Sepharose (50 μl of 50% bead slurry). After quick centrifugation, the pellets are washed in lysis buffer containing increasing salt concentration (e.g., 150, 300, 500 mM). The beads are then re-equilibrated at 150 mM NaCl before addition of SDS-containing sample buffer and boiling. After centrifugation, the supernatants are optionally electrophoresed using 4%-12% gradient Bis-Tris gels followed by transfer into Immobilon-P membranes. After blocking, blots are optionally incubated with an antibody that detects FITC and also with one or more antibodies that detect proteins that bind to the peptidomimetic macrocycle.
Cellular Penetrability Assays.
[0492]To measure the cell penetrability of peptidomimetic macrocycles and corresponding uncrosslinked macrocycle, intact cells are incubated with fluoresceinated peptidomimetic macrocycles or corresponding uncrosslinked macrocycle (10 μM) for 4 hrs in serum free media at 37° C., washed twice with media and incubated with trypsin (0.25%) for 10 min at 37° C. The cells are washed again and resuspended in PBS. Cellular fluorescence is analyzed, for example, by using either a FACSCalibur flow cytometer or Cellomics' KineticScan® HCS Reader.
In Vivo Stability Assays.
[0493]To investigate the in vivo stability of the peptidomimetic macrocycles, the compounds are, for example, administered to mice or rats by IV, IP, PO or inhalation routes at concentrations ranging from 0.1 to 50 mg/kg and blood specimens withdrawn at 0′, 5′, 15′, 30′, 1 hr, 4 hrs, 8 hrs and 24 hours post-injection. Levels of intact compound in 25 μL of fresh serum are then measured by LC-MS/MS as above.
Clinical Trials.
[0494]To determine the suitability of the peptidomimetic macrocycles of the invention for treatment of humans, clinical trials are performed. For example, patients diagnosed with a muscle wasting disease or lipodystrophy and in need of treatment are selected and separated in treatment and one or more control groups, wherein the treatment group is administered a peptidomimetic macrocycle of the invention, while the control groups receive a placebo or a known BH3 mimetic. The treatment safety and efficacy of the peptidomimetic macrocycles of the invention can thus be evaluated by performing comparisons of the patient groups with respect to factors such as survival and quality-of-life. In this example, the patient group treated with a peptidomimetic macrocycle show improved long-term survival compared to a patient control group treated with a placebo.
Pharmaceutical Compositions and Routes of Administration
[0495]In some embodiments, the present invention provides a pharmaceutical composition comprising a peptidomimetic macrocycle of the invention and a pharmaceutically acceptable carrier.
[0496]The peptidomimetic macrocycles of the invention also include pharmaceutically acceptable derivatives or prodrugs thereof. A “pharmaceutically acceptable derivative” means any pharmaceutically acceptable salt, ester, salt of an ester, pro-drug or other derivative of a compound of this invention which, upon administration to a recipient, is capable of providing (directly or indirectly) a compound of this invention. Particularly favored pharmaceutically acceptable derivatives are those that increase the bioavailability of the compounds of the invention when administered to a mammal (e.g., by increasing absorption into the blood of an orally administered compound) or which increases delivery of the active compound to a biological compartment (e.g., the brain or lymphatic system) relative to the parent species. Some pharmaceutically acceptable derivatives include a chemical group which increases aqueous solubility or active transport across the gastrointestinal mucosa.
[0497]In some embodiments, the peptidomimetic macrocycles of the invention are modified by covalently or non-covalently joining appropriate functional groups to enhance selective biological properties. Such modifications include those which increase biological penetration into a given biological compartment (e.g., blood, lymphatic system, central nervous system), increase oral availability, increase solubility to allow administration by injection, alter metabolism, and alter rate of excretion.
[0498]Pharmaceutically acceptable salts of the compounds of this invention include those derived from pharmaceutically acceptable inorganic and organic acids and bases. Examples of suitable acid salts include acetate, adipate, benzoate, benzenesulfonate, butyrate, citrate, digluconate, dodecylsulfate, formate, fumarate, glycolate, hemisulfate, heptanoate, hexanoate, hydrochloride, hydrobromide, hydroiodide, lactate, maleate, malonate, methanesulfonate, 2-naphthalenesulfonate, nicotinate, nitrate, palmoate, phosphate, picrate, pivalate, propionate, salicylate, succinate, sulfate, tartrate, tosylate and undecanoate. Salts derived from appropriate bases include alkali metal (e.g., sodium), alkaline earth metal (e.g., magnesium), ammonium and N-(alkyl)4+ salts.
[0499]For preparing pharmaceutical compositions from the compounds of the present invention, pharmaceutically acceptable carriers include either solid or liquid carriers. Solid form preparations include powders, tablets, pills, capsules, cachets, suppositories, and dispersible granules. A solid carrier can be one or more substances, which also acts as diluents, flavoring agents, binders, preservatives, tablet disintegrating agents, or an encapsulating material. Details on techniques for formulation and administration are well described in the scientific and patent literature, see, e.g., the latest edition of Remington's Pharmaceutical Sciences, Maack Publishing Co, Easton Pa.
[0500]In powders, the carrier is a finely divided solid, which is in a mixture with the finely divided active component. In tablets, the active component is mixed with the carrier having the necessary binding properties in suitable proportions and compacted in the shape and size desired.
[0501]Suitable solid excipients are carbohydrate or protein fillers include, but are not limited to sugars, including lactose, sucrose, mannitol, or sorbitol; starch from corn, wheat, rice, potato, or other plants; cellulose such as methyl cellulose, hydroxypropylmethyl-cellulose, or sodium carboxymethylcellulose; and gums including arabic and tragacanth; as well as proteins such as gelatin and collagen. If desired, disintegrating or solubilizing agents are added, such as the cross-linked polyvinyl pyrrolidone, agar, alginic acid, or a salt thereof, such as sodium alginate.
[0502]Liquid form preparations include solutions, suspensions, and emulsions, for example, water or water/propylene glycol solutions. For parenteral injection, liquid preparations can be formulated in solution in aqueous polyethylene glycol solution.
[0503]The pharmaceutical preparation is preferably in unit dosage form. In such form the preparation is subdivided into unit doses containing appropriate quantities of the active component. The unit dosage form can be a packaged preparation, the package containing discrete quantities of preparation, such as packeted tablets, capsules, and powders in vials or ampoules. Also, the unit dosage form can be a capsule, tablet, cachet, or lozenge itself, or it can be the appropriate number of any of these in packaged form.
[0504]When the compositions of this invention comprise a combination of a peptidomimetic macrocycle and one or more additional therapeutic or prophylactic agents, both the compound and the additional agent should be present at dosage levels of between about 1 to 100%, and more preferably between about 5 to 95% of the dosage normally administered in a monotherapy regimen. In some embodiments, the additional agents are administered separately, as part of a multiple dose regimen, from the compounds of this invention. Alternatively, those agents are part of a single dosage form, mixed together with the compounds of this invention in a single composition.
[0505]In some embodiments, the compositions are present as unit dosage forms that can deliver, for example, from about 0.0001 mg to about 1,000 mg of the peptidomimetic macrocycles, salts thereof, prodrugs thereof, derivatives thereof, or any combination of these. Thus, the unit dosage forms can deliver, for example, in some embodiments, from about 1 mg to about 900 mg, from about 1 mg to about 800 mg, from about 1 mg to about 700 mg, from about 1 mg to about 600 mg, from about 1 mg to about 500 mg, from about 1 mg to about 400 mg, from about 1 mg to about 300 mg, from about 1 mg to about 200 mg, from about 1 mg to about 100 mg, from about 1 mg to about 10 mg, from about 1 mg to about 5 mg, from about 0.1 mg to about 10 mg, from about 0.1 mg to about 5 mg, from about 10 mg to about 1,000 mg, from about 50 mg to about 1,000 mg, from about 100 mg to about 1,000 mg, from about 200 mg to about 1,000 mg, from about 300 mg to about 1,000 mg, from about 400 mg to about 1,000 mg, from about 500 mg to about 1,000 mg, from about 600 mg to about 1,000 mg, from about 700 mg to about 1,000 mg, from about 800 mg to about 1,000 mg, from about 900 mg to about 1,000 mg, from about 10 mg to about 900 mg, from about 100 mg to about 800 mg, from about 200 mg to about 700 mg, or from about 300 mg to about 600 mg of the peptidomimetic macrocycles, salts thereof, prodrugs thereof, derivatives thereof, or any combination of these.
[0506]In some embodiments, the compositions are present as unit dosage forms that can deliver, for example, about 1 mg, about 2 mg, about 3 mg, about 4 mg, about 5 mg, about 6 mg, about 7 mg, about 8 mg, about 9 mg, about 10 mg, about 20 mg, about 30 mg, about 40 mg, about 50 mg, about 60 mg, about 70 mg, about 80 mg, about 90 mg, about 100 mg, about 150 mg, about 200 mg, about 250 mg, about 300 mg, about 350 mg, about 400 mg, about 500 mg, about 600 mg, about 700 mg, about 800 mg, or about 1000 mg of peptidomimetic macrocycles, salts thereof, prodrugs thereof, derivatives thereof, or any combination of these.
[0507]Suitable routes of administration include, but are not limited to, oral, intravenous, rectal, aerosol, parenteral, ophthalmic, pulmonary, transmucosal, transdermal, vaginal, otic, nasal, and topical administration. In addition, by way of example only, parenteral delivery includes intramuscular, subcutaneous, intravenous, intramedullary injections, as well as intrathecal, direct intraventricular, intraperitoneal, intralymphatic, and intranasal injections.
[0508]In certain embodiments, a composition as described herein is administered in a local rather than systemic manner, for example, via injection of the compound directly into an organ. In specific embodiments, long acting formulations are administered by implantation (for example subcutaneously or intramuscularly) or by intramuscular injection. Furthermore, in other embodiments, the drug is delivered in a targeted drug delivery system, for example, in a liposome coated with organ-specific antibody. In such embodiments, the liposomes are targeted to and taken up selectively by the organ. In yet other embodiments, the compound as described herein is provided in the form of a rapid release formulation, in the form of an extended release formulation, or in the form of an intermediate release formulation. In yet other embodiments, the compound described herein is administered topically.
[0509]In another embodiment, compositions described herein are formulated for oral administration. Compositions described herein are formulated by combining a peptidomimetic macrocycle with, e.g., pharmaceutically acceptable carriers or excipients. In various embodiments, the compounds described herein are formulated in oral dosage forms that include, by way of example only, tablets, powders, pills, dragees, capsules, liquids, gels, syrups, elixirs, slurries, suspensions and the like.
[0510]In certain embodiments, pharmaceutical preparations for oral use are obtained by mixing one or more solid excipient with one or more of the peptidomimetic macrocycles described herein, optionally grinding the resulting mixture, and processing the mixture of granules, after adding suitable auxiliaries, if desired, to obtain tablets or dragee cores. Suitable excipients are, in particular, fillers such as sugars, including lactose, sucrose, mannitol, or sorbitol; cellulose preparations such as: for example, maize starch, wheat starch, rice starch, potato starch, gelatin, gum tragacanth, methylcellulose, microcrystalline cellulose, hydroxypropylmethylcellulose, sodium carboxymethylcellulose; or others such as: polyvinylpyrrolidone (PVP or povidone) or calcium phosphate. In specific embodiments, disintegrating agents are optionally added. Disintegrating agents include, by way of example only, cross-linked croscarmellose sodium, polyvinylpyrrolidone, agar, or alginic acid or a salt thereof such as sodium alginate.
[0511]In one embodiment, dosage forms, such as dragee cores and tablets, are provided with one or more suitable coating. In specific embodiments, concentrated sugar solutions are used for coating the dosage form. The sugar solutions, optionally contain additional components, such as by way of example only, gum arabic, talc, polyvinylpyrrolidone, carbopol gel, polyethylene glycol, or titanium dioxide, lacquer solutions, and suitable organic solvents or solvent mixtures. Dyestuffs or pigments are also optionally added to the coatings for identification purposes. Additionally, the dyestuffs or pigments are optionally utilized to characterize different combinations of active compound doses.
[0512]In certain embodiments, therapeutically effective amounts of at least one of the peptidomimetic macrocycles described herein are formulated into other oral dosage forms. Oral dosage forms include push-fit capsules made of gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer, such as glycerol or sorbitol. In specific embodiments, push-fit capsules contain the active ingredients in admixture with one or more filler. Fillers include, by way of example only, lactose, binders such as starches, or lubricants such as talc or magnesium stearate and, optionally, stabilizers. In other embodiments, soft capsules, contain one or more active compound that is dissolved or suspended in a suitable liquid. Suitable liquids include, by way of example only, one or more fatty oil, liquid paraffin, or liquid polyethylene glycol. In addition, stabilizers are optionally added.
[0513]In other embodiments, therapeutically effective amounts of at least one of the peptidomimetic macrocycles described herein are formulated for buccal or sublingual administration. Formulations suitable for buccal or sublingual administration include, by way of example only, tablets, lozenges, or gels. In still other embodiments, the peptidomimetic macrocycles described herein are formulated for parenteral injection, including formulations suitable for bolus injection or continuous infusion. In specific embodiments, formulations for injection are presented in unit dosage form (e.g., in ampoules) or in multi-dose containers. Preservatives are, optionally, added to the injection formulations. In still other embodiments, pharmaceutical compositions are formulated in a form suitable for parenteral injection as a sterile suspensions, solutions or emulsions in oily or aqueous vehicles. Parenteral injection formulations optionally contain formulatory agents such as suspending, stabilizing or dispersing agents. In specific embodiments, pharmaceutical formulations for parenteral administration include aqueous solutions of the active compounds in water-soluble form. In additional embodiments, suspensions of the active compounds are prepared as appropriate oily injection suspensions. Suitable lipophilic solvents or vehicles for use in the pharmaceutical compositions described herein include, by way of example only, fatty oils such as sesame oil, or synthetic fatty acid esters, such as ethyl oleate or triglycerides, or liposomes. In certain specific embodiments, aqueous injection suspensions contain substances which increase the viscosity of the suspension, such as sodium carboxymethyl cellulose, sorbitol, or dextran. Optionally, the suspension contains suitable stabilizers or agents which increase the solubility of the compounds to allow for the preparation of highly concentrated solutions. Alternatively, in other embodiments, the active ingredient is in powder form for constitution with a suitable vehicle, e.g., sterile pyrogen-free water, before use.
[0514]Pharmaceutical compositions herein can be administered, for example, once or twice or three or four or five or six times per day, or once or twice or three or four or five or six times per week, and can be administered, for example, for a day, a week, a month, 3 months, six months, a year, five years, or for example ten years. In some embodiments, a pharmaceutical formulation of the invention is administered no more frequently than once daily, no more frequently than every other day, no more frequently than twice weekly, no more frequently than three times weekly, no more frequently than four times weekly, no more frequently than five times weekly, or no more frequently than every other week. In some embodiments, a pharmaceutical formulation of the invention is administered no more than once weekly. In some embodiments, a pharmaceutical formulation of the invention is administered no more than twice weekly. In some embodiments, a pharmaceutical formulation of the invention is administered no more than three times weekly. In some embodiments, a pharmaceutical formulation of the invention is administered no more than four times weekly. In some embodiments, a pharmaceutical formulation of the invention is administered no more than five times weekly.
Methods of Use
[0515]As used herein, the term “treatment” is defined as the application or administration of a therapeutic agent to a patient, or application or administration of a therapeutic agent to an isolated tissue or cell line from a patient, who has a disease, a symptom of disease or a predisposition toward a disease, with the purpose to cure, heal, alleviate, relieve, alter, remedy, ameliorate, improve or affect the disease, the symptoms of disease or the predisposition toward disease. In some embodiments, a peptidomimetic macrocycle disclosed herein is used for treating a disease or condition in a subject in need thereof. In some embodiments, a peptidomimetic macrocycle disclosed herein is used for manufacture of a medicament for treating a disease or condition in a subject in need thereof.
[0516]In one aspect, the present invention provides novel peptidomimetic macrocycles that are useful in competitive binding assays to identify agents which bind to a natural ligand of the proteins or peptides upon which the peptidomimetic macrocycles are modeled. For example, labeled peptidomimetic macrocycles based on BIM can be used in a binding assay along with small molecules that competitively bind to BFL-1 or a BCL-2 family protein. Competitive binding studies allow for rapid in vitro evaluation and determination of drug candidates specific to the BIM/BFL-1 or a BCL-2 family protein interaction. Such binding studies may be performed with any of the peptidomimetic macrocycles disclosed herein and their binding partners.
[0517]The invention further provides for the generation of antibodies against the peptidomimetic macrocycles. In some embodiments, these antibodies specifically bind both the peptidomimetic macrocycle and the precursor peptides, such as BIM, to which the peptidomimetic macrocycles are related. Such antibodies, for example, disrupt the native protein-protein interactions, for example, between BIM and BFL-1 or a BCL-2 family protein.
[0518]In another aspect, the present invention provides methods to inhibit BFL-1 or a BCL-2 family protein, thereby stimulating death of a cell or tissue. In some embodiments, a subject suffering from a condition of suppressed cell death, such as B-cell lymphoma, is treated using pharmaceutical compositions of the invention.
[0519]In yet another aspect, the present invention provides methods for treating a disease driven by over-expression of BFL-1 or a BCL-2 family protein. In some embodiments, the disease driven by over-expression is a cancer. The cancer can be a liquid cancer or a solid cancer. Non-limiting examples of a liquid cancer include leukemia, lymphoma, myeloma, and myeloid dysplasia. Non-limiting examples of a solid cancer include lung cancer, breast cancer, colon cancer, brain cancer, liver cancer, soft-tissue sarcoma, pancreatic cancer, and melanoma. In some embodiments, the cancer is resistant, non-responsive, or determined unlikely to respond to a BCL-2 inhibitor.
[0520]In some embodiments, the compounds of the present invention are administered in combination with a second therapeutic agent. In some embodiments, the compounds of the present invention are administered with compounds that inhibit the activity of BCL-2 anti-apoptotic proteins. In some embodiments, the BCL-2 inhibitor is a BH3 mimetic. In some embodiments, the BCL-2 inhibitor is navitoclax (ABT-263), obatoclax (GX15-070), or venetoclax. These methods comprise administering an effective amount of a compound of the invention to a warm blooded animal, including a human. In some embodiments, a pharmaceutical composition provided herein used in the treatment of a BFL-1 over-expressing cancer is administered no more frequently than once daily, no more frequently than every other day, no more frequently than twice weekly, no more frequently than weekly, or no more frequently than every other week.
[0521]In some embodiments, provided herein are methods for treating neurodegenerative disorders. Many neurodegenerative diseases are a result of neurodegenerative processes including progressive loss of structure or function of neurons. These methods comprise administering an effective amount of at least one peptidomimetic macrocycles of the invention or a pharmaceutical composition thereof to a warm blooded animal, including a human. Non limiting neurodegenerative disorders that may be treated by the methods of the present invention include Parkinson's disease, Alzheimer's, Amyotrophic lateral sclerosis (ALS) and Huntington's disease.
[0522]In some embodiments, provided herein are methods for treating cardiac disorders. These methods comprise administering an effective amount of at least one peptidomimetic macrocycles of the invention or a pharmaceutical composition thereof to a warm blooded animal, including a human. Non limiting examples of cardiac disorders that may be treated by the methods of the present invention include coronary heart disease (also known as isohaemic heart disease or coronary artery disease), cardiomyopathy (diseases of cardiac muscle), hypertensive heart disease (diseases of the heart secondary to high blood pressure), heart failure, cor pulmonale (failure of the right side of the heart), cardiac dysrhythmias (abnormalities of heart rhythm), inflammatory heart disease, endocarditis (inflammation of the inner layer of the heart, the endocardium), inflammatory cardiomegaly, myocarditis (inflammation of the myocardium, the muscular part of the heart), valvular heart disease, cerebrovascular disease (disease of blood vessels that supplies to the brain such as stroke), peripheral arterial disease (disease of blood vessels that supplies to the arms and legs), congenital heart disease, and rheumatic heart disease. In some embodiments, the methods of the present invention may be used for the treatment of acute myocardial infarction or chromic ischemic heart disease.
[0523]Also provided herein are methods for promoting cardiac regeneration in a subject in need thereof. These methods comprise administering an effective amount of at least one peptidomimetic macrocycles of the invention or a pharmaceutical composition thereof to a warm blooded animal, including a human.
[0524]In some embodiments, provided herein are methods for treating diabetes or diabetes mellitus. Diabetes is a group of metabolic diseases in which a person has high blood sugar, either because the pancreas does not produce enough insulin, or because cells do not respond to the insulin that is produced. The diabetes may be Type 1 diabetes mellitus, type 2 diabetes, gestational diabetes, congenital diabetes, cystic fibrosis-related diabetes or several forms of monogenic diabetes. Treatment of diabetes may be by islet/beta cell transplantation.
[0525]In another aspect the invention provides methods of treating a subject by administering to the subject a beta cell, wherein the beta cell has been treated with an effective amount of a peptidomimetic macrocycle of the invention or a pharmaceutical composition thereof. Similarly, In another aspect the invention provides methods of treating a subject by administering to the subject a islet cell, wherein the islet cell has been treated with an effective amount of a peptidomimetic macrocycle of the invention or a pharmaceutical composition thereof.
[0526]In some embodiments, provided herein are methods for treating cancer. These methods comprise administering an effective amount of at least one peptidomimetic macrocycles of the invention or a pharmaceutical composition thereof to a warm blooded animal, including a human. Non-limiting examples of cancers that may be treated by the methods of the present invention include breast cancer such as a ductal carcinoma in duct tissue in a mammary gland, medullary carcinomas, colloid carcinomas, tubular carcinomas, and inflammatory breast cancer; ovarian cancer, including epithelial ovarian tumors such as adenocarcinoma in the ovary and an adenocarcinoma that has migrated from the ovary into the abdominal cavity; uterine cancer; cervical cancer such as adenocarcinoma in the cervix epithelial including squamous cell carcinoma and adenocarcinomas; prostate cancer, such as a prostate cancer selected from the following: an adenocarcinoma or an adenocarcinoma that has migrated to the bone; pancreatic cancer such as epithelioid carcinoma in the pancreatic duct tissue and an adenocarcinoma in a pancreatic duct; bladder cancer such as a transitional cell carcinoma in urinary bladder, urothelial carcinomas (transitional cell carcinomas), tumors in the urothelial cells that line the bladder, squamous cell carcinomas, adenocarcinomas, and small cell cancers; leukemia such as acute myeloid leukemia (AML), acute lymphocytic leukemia, chronic lymphocytic leukemia, chronic myeloid leukemia, hairy cell leukemia, myelodysplasia, myeloproliferative disorders, acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML), mastocytosis, chronic lymphocytic leukemia (CLL), multiple myeloma (MM), and myelodysplastic syndrome (MDS); bone cancer; lung cancer such as non-small cell lung cancer (NSCLC), which is divided into squamous cell carcinomas, adenocarcinomas, and large cell undifferentiated carcinomas, and small cell lung cancer; skin cancer such as basal cell carcinoma, melanoma, squamous cell carcinoma and actinic keratosis, which is a skin condition that sometimes develops into squamous cell carcinoma; eye retinoblastoma; cutaneous or intraocular (eye) melanoma; primary liver cancer (cancer that begins in the liver); kidney cancer; thyroid cancer such as papillary, follicular, medullary and anaplastic; AIDS-related lymphoma such as diffuse large B-cell lymphoma, B-cell immunoblastic lymphoma and small non-cleaved cell lymphoma; Kaposi's Sarcoma; viral-induced cancers including hepatitis B virus (HBV), hepatitis C virus (HCV), and hepatocellular carcinoma; human lymphotropic virus-type 1 (HTLV-1) and adult T-cell leukemia/lymphoma; and human papilloma virus (HPV) and cervical cancer; central nervous system cancers (CNS) such as primary brain tumor, which includes gliomas (astrocytoma, anaplastic astrocytoma, or glioblastoma multiforme), Oligodendroglioma, Ependymoma, Meningioma, Lymphoma, Schwannoma, and Medulloblastoma; peripheral nervous system (PNS) cancers such as acoustic neuromas and malignant peripheral nerve sheath tumor (MPNST) including neurofibromas and schwannomas, malignant fibrous cytoma, malignant fibrous histiocytoma, malignant meningioma, malignant mesothelioma, and malignant mixed Müllerian tumor; oral cavity and oropharyngeal cancer such as, hypopharyngeal cancer, laryngeal cancer, nasopharyngeal cancer, and oropharyngeal cancer; stomach cancer such as lymphomas, gastric stromal tumors, and carcinoid tumors; testicular cancer such as germ cell tumors (GCTs), which include seminomas and nonseminomas, and gonadal stromal tumors, which include Leydig cell tumors and Sertoli cell tumors; thymus cancer such as to thymomas, thymic carcinomas, Hodgkin disease, non-Hodgkin lymphomas carcinoids or carcinoid tumors; rectal cancer; and colon cancer.
[0527]In some embodiments, a peptidomimetic macrocycle disclosed herein is administered in combination with an additional therapy to treat a cancer. Non-limiting examples of the additional therapy include surgery, radiation therapy, chemotherapy, or immunotherapy. In some embodiments, the combination of the peptidomimetic macrocycle and surgery is on an adjuvant basis or a neo-adjuvant basis.
[0528]Non-limiting examples of chemotherapy include alkylating agents, angiogenesis inhibitors, antimetabolites, Bcr-Abl kinase inhibitors, cyclin-dependent kinase inhibitors, cyclooxygenase-2 inhibitors, epidermal growth factor receptor (EGFR) inhibitors, leukemia viral oncogene homolog (ErbB2) receptor inhibitors, histone deacetylase (HDAC) inhibitors, heat shock protein (HSP)-90 inhibitors, inhibitors of inhibitors of apoptosis proteins (IAPs), antibody drug conjugates, activators of death receptor pathway, kinesin inhibitors, JAK-2 inhibitors, mitogen-activated extracellular signal-regulated kinase (MEK) inhibitors, mammalian target of rapamycin (mTOR) inhibitors, non-steroidal anti-inflammatory drugs (NSAIDs), platelet-derived growth factor receptor (PDGFR) inhibitors, platinum chemotherapeutics, polo-like kinase (Plk) inhibitors, phosphoinositide-3 kinase (PI3K) inhibitors, thrombospondin analogues, vascular endothelial growth factor receptor (VEGFR) inhibitors, intercalating antibiotics, topoisomerase inhibitors, antibodies, hormonal therapies, deltoids and retinoids, poly ADP (adenosine diphosphate)-ribose polymerase (PARP) inhibitors, plant alkaloids, proteasome inhibitors, biologic response modifiers, pyrimidine analogues, purine analogues, antimitotics, taxanes, and ubiquitin ligase inhibitors.
[0529]Non-limiting examples of alkylating agents include: altretamine, AMD-473, AP-5280, apaziquone, bendamustine, brostallicin, busulfan, carboquone, carmustine, chlorambucil, laromustine, cyclophosphamide, decarbazine, estramustine, fotemustine, glufosfamide, ifosfamide, KW-2170, lomustine, mafosfamide, melphalan, mitobronitol, mitolactol, nimustine, nitrogen mustard N-oxide, ranimustine, temozolomide, thiotepa, bendamustine, treosulfan, and rofosfamide.
[0530]Non-limiting examples of angiogenesis inhibitors include: endothelial-specific receptor tyrosine kinase (Tie-2) inhibitors, epidermal growth factor receptor (EGFR) inhibitors, insulin growth factor-2 receptor (IGFR-2) inhibitors, matrix metalloproteinase-2 (MMP-2) inhibitors, matrix metalloproteinase-9 (MMP-9) inhibitors, platelet-derived growth factor receptor (PDGFR) inhibitors, thrombospondin analogues, and vascular endothelial growth factor receptor tyrosine kinase (VEGFR) inhibitors.
[0531]Non-limiting examples of antimetabolites include: pemetrexed disodium, 5-azacitidine, capecitabine, carmofur, cladribine, clofarabine, cytarabine, cytarabine ocfosfate, cytosine arabinoside, decitabine, deferoxamine, doxifluridine, eflornithine, EICAR, enocitabine, ethnylcytidine, fludarabine, 5-fluorouracil, leucovorin, gemcitabine, hydroxyurea, melphalan, mercaptopurine, 6-mercaptopurine riboside, methotrexate, mycophenolic acid, nelarabine, nolatrexed, ocfosfate, pelitrexol, pentostatin, raltitrexed, Ribavirin, triapine, trimetrexate, S-1, tiazofurin, tegafur, TS-1, vidarabine, and UFT.
[0532]Non-limiting examples of Bcr-Abl kinase inhibitors include: dasatinib, nilotinib, and imatinib.
[0533]Non-limiting examples of CDK inhibitors include: AZD-5438, BMI-1040, BMS-032, BMS-387, CVT-2584, flavopyridol, GPC-286199, MCS-5A, PD0332991, PHA-690509, seliciclib, and ZK-304709.
[0534]Non-limiting examples of COX-2 inhibitors include: ABT-963, etoricoxib, valdecoxib, BMS347070, celecoxib, lumiracoxib, CT-3, deracoxib, JTE-522, 4-methy dimethylphenyl)-1-(4-sulfamoylphenyl-1H-pyrrole), etoricoxib, NS-398, parecoxib, RS-57067, SC-58125, SD-8381, SVT-2016, S-2474. T-614, and rofecoxib.
[0535]Non-limiting examples of EGFR inhibitors include: ABX-EGF, anti-EGFR immunoliposomes, EGF-vaccine, EMD-7200, cetuximab, IgA antibodies, gefitinib, erlotinib, TP-38, EGFR fusion protein, and lapatinib.
[0536]Non-limiting examples of ErbB2 receptor inhibitors include: CP-724-714, canertinib, trastuzumab, lapatinib, petuzumab, TAK-165, ionafarnib, GW-282974, EKB-569, PI-166, dHER2 HER2. vaccine, APC-8024 HER-2 vaccine, anti-HER2/neu bispecific antibody, B7.her2IgG3, AS HER2 trifunctional bispecific antibodies, mAB AR-209, and mAB 2B-1.
[0537]Non-limiting examples of histone deacetylase inhibitors include: depsipeptide, LAQ-824, MS-275, trapoxin, suberoylanilide hydroxamic acid (SAHA), TSA, and valproic acid.
[0538]Non-limiting examples of HSP-90 inhibitors include: 17-AAG-nab, 17-AAG, CNF-101, CNF-1010, CNF-2024, 17-DMAG, geldanamycin, IPI-504, KOS-953, human recombinant antibody to HSP-90, NCS-683664, PU24FC1, PU-3, radicicol, SNX-2112, or STA-9090 VER49009,
[0539]Non-limiting examples of inhibitors of inhibitors of apoptosis proteins include: HGS1029, GDC-0145, GDC-0152, LCL-161, and LBW-242.
[0540]Non-limiting examples of antibody-drug conjugates include: anti-CD22-MC-MMAF, anti-CD22-MC-MMAE, anti-CD22-MCC-DM1, CR-0,1-vcMMAE, PSMA-ADC, MEDI-547, SGN-19Am SGN-35, and SGN-75.
[0541]Non-limiting examples of activators of death receptor pathway include: TRAIL, antibodies or other agents that target TRAIL or death receptors (e.g., DR4 and DR5) such as apomab, conatumumab, ETR2-ST01, GDC0145, lexatumumab, HGS-1029, LBY-135, PRO-1762, and trastuzumab.
[0542]Non-limiting; examples of kinesin inhibitors include: Eg5 inhibitors such as AZD4877, ARRY-520; and CENPE inhibitors such as GSK923295A.
[0543]Non-limiting examples of JAK-2 inhibitors include: lesaurtinib, XL019 or INCB018424.
[0544]Non-limiting examples of MEK inhibitors include: trametinib, ARRY-142886, ARRY-438162 PD-325901, CI-1040, and PD-98059.
[0545]Non-limiting examples of mTOR inhibitors include: AP-23573, CCI-779, everolimus, RAD-001, rapamycin, temsirolimus, ATP-competitive. TORC1/TORC2 inhibitors, comprising P1-103, PP242, PP30, and Torin 1.
[0546]Non-limiting examples of non-steroidal anti-inflammatory drugs include: salsalate, diflunisal, ibuprofen, ketoprofen, nabumetone, piroxicam, ibuprofen cream, naproxen, diclofenac, indomethacin, sulindac, tolmetin, etodolac, ketorolac, and oxaprozin.
[0547]Non-limiting examples of PDGFR inhibitors include: C-451, CP-673, and CP-868596.
[0548]Non-limiting examples of platinum chemotherapeutics include: cisplatin, eptaplatin, lobaplatin, nedaplatin, carboplatin, satraplatin, and picoplatin.
[0549]Non-limiting examples of polo-like kinase inhibitors include: BI-2536.
[0550]Non-limiting examples of phosphoinositide-3 kinase (MK) inhibitors include: wortmannin, LY294002, XL-147, CAL-120, ONC-21, AEZS-127, ETP-45658, PX-866, GDC-0941, BGT226, BEZ235, and XL765.
[0551]Non-limiting examples of thrombospondin analogues include: ABT-510, ABT-567, ABT-898, and TSP-1.
[0552]Non-limiting examples of VEGFR inhibitors include: bevacizumab, ABT-869, AEE-788, ANGIOZYME™ (a ribozyme that inhibits angiogenesis, axitinib, AZD-2171, CP-547,632, IM-862, pegaptamib, sorafenib, pazopanib, vatalanib, sunitinib, VEGF trap, and vandetanib.
[0553]Non-limiting examples of antibiotics include: intercalating antibiotics aclarubicin, actinomycin amrubicin, annamycin, adriamycin, bleomycin, daunorubicin, liposomal doxorubicin, doxorubicin, elsamitrucin, epirbucin, glarbuicin, idarubicin, mitomycin C, nemorubicin, neocarzinostatin, peplomycin, pirarubicin, rebeccamycin, stimalamer, streptozocin, vairubicin, and zinostatin.
[0554]Non-limiting examples of topoisomerase inhibitors include: aclarubicin, 9-aminocamptothecin, amonafide, amsacrine, becatecarin, belotecan, BN-80915, irinotecan, camptothecin, dexrazoxine, diflomotecan, edotecarin, epirubicin, etoposide, exatecan, 10-hydroxycamptothecin, gimatecan, lurtotecan, mitoxantrone, orathecin, pirarbucin, pixantrone, rubitecan, sobuzoxane, SN-38, tafluposide, and topotecan.
[0555]Non-limiting examples of antibodies include: bevacizumab, CD40 antibodies, chTNT-1/B, denosumab, cetuximab, zanolimumab, IGF1R antibodies, lintuzumab, edrecolomab, WX G250, rituximab, ticilimumab, trastuzumab, CD20 antibodies types I and II, pernbrolizumab, nivolumab, rituximab, and panitumumab.
[0556]Non-limiting examples of hormonal therapies include: anastrozole, exemestane, arzoxifene, bicalutamide, cetrorelix, degarelix, deslorelin, trilostane, dexamethasone, flutamide, raloxifene, fadrozole, toremifene, fulvestrant, letrozole, formestane, glucocorticoids, doxercalciferol, sevelamer carbonate, lasofoxifene, leuprolide acetate, megesterol, mifepristone, nilutamide, tamoxifen citrate, abarelix, prednisone, finasteride, rilostane, buserelin, luteinizing hormone releasing hormone (TA-IRA), histrelin implant, trilostane, modrastane, fosrelin, and goserelin.
[0557]Non-limiting examples of deltoids and retinoids include: seocalcitol, lexacalcitrol, fenretinide, aliretinoin, liposomal tretinoin, bexarotene, and LGD-1550.
[0558]Non-limiting examples of PARP inhibitors include: ABT-888, olaparib, KU-59436, AZD-2281 AG-014699, BSI-201, BGP-15, INO-1001, and ONO-2231.
[0559]Non-limiting examples of plant alkaloids include: vincristine, vinblastine, vindesine, and vinorelbine.
[0560]Non-limiting examples of proteasome inhibitors include: bortezomib, carfilzomib, MG132, and NPI-0052.
[0561]Non-limiting examples of biological response modifiers include: krestin, sizofuran, picibanil, PF-3512676, and ubenimex.
[0562]Non-limiting examples of pyrimidine analogues include: cytarabine, cytosine arabinoside, doxifluridine, fludarabine, 5-fluorouracil, floxuridine, gemcitabine, ratitrexed, and triacetvluridine troxacitabine.
[0563]Non-limiting examples of purine analogues include: thioguanine, and mercaptopurine.
[0564]Non-limiting examples of antimitotic agents include: batabulin, epothilone D, N-(2-((4-hydroxyphenyl)amino)pyridin-3-yl)-4-methoxybenzenesulfonamide, ixabepilone, paclitaxel, docetaxel, PNU100940, patupilone, XRP-9881 larotaxel, vinflunine, and epothilone.
[0565]Non-limiting examples of ubiquitin ligase inhibitors include paclitaxel and docetaxel.
[0566]Non-limiting examples of ubiquitin ligase inhibitors include: MDM2 inhibitors, such as nutlins, and NEDD8 inhibitors such as MLN4924.
[0567]Non-limiting examples of immunotherapies include: interferons or immune-enhancing agents. Interferons comprise interferon alpha, interferon alpha-2a, interferon alpha-2h, interferon beta, interferon gamma-1a, interferon gamma-1b, interferon gamma-n1. Other immune-enhancing agents comprise oxidized glutathione, tasonermin, tositumomab, alemtuzumab, CTLA4, decarbazine, denileukin, epratuzumab, lenograstim, lentinan, leukocyte alpha interferon, imiquimod, ipilumimab, melanoma vaccine, mitumomab, molgramostim, nivolumab, pembrolizumab, gemtuzumab ozogamicin, filgrastim, OncoVAC-CL, oregovomab, pemtumomab, sipuleucel-T, sargaramostim, sizofilan, teceleukin, Bacillus Calmette-Guerin, ubenimex, virulizin, Z-100, Tetrachlorodecaoxide (TCDD), aldesleukin, thymalfasin, daclizumab, and 90Y-Ibritumomab tiuxetan.
[0568]While preferred embodiments of the present invention have been shown and described herein, it will be obvious to those skilled in the art that such embodiments are provided by way of example only. Numerous variations, changes, and substitutions will now occur to those skilled in the art without departing from the invention. It should be understood that various alternatives to the embodiments of the invention described herein may be employed in practicing the invention. It is intended that the following claims define the scope of the invention and that methods and structures within the scope of these claims and their equivalents be covered thereby.
EXAMPLES
Example 1: Peptidomimetic Macrocycles of the Invention
[0569]Peptidomimetic macrocycles were synthesized, purified and analyzed as previously described and as described below (Schafmeister et al., J. Am. Chem. Soc. 122:5891-5892 (2000); Schafmeister & Verdin, J. Am. Chem. Soc. 122:5891 (2005); Walensky et al., Science 305:1466-1470 (2004); and U.S. Pat. No. 7,192,713). Peptidomimetic macrocycles were designed by replacing two or more naturally occurring amino acids with the corresponding synthetic amino acids. Substitutions were made at i and i+4, and i and i+7 positions. Peptide synthesis was performed either manually or on an automated peptide synthesizer (Applied Biosystems, model 433A), using solid phase conditions, rink amide AM resin (Novabiochem), and Fmoc main-chain protecting group chemistry. For the coupling of natural Fmoc-protected amino acids (Novabiochem), 10 equivalents of amino acid and a 1:1:2 molar ratio of coupling reagents HBTU/HOBt (Novabiochem)/DIEA were employed. Non-natural amino acids (4 equiv) were coupled with a 1:1:2 molar ratio of HATU (Applied Biosystems)/HOBt/DIEA. The N-termini of the synthetic peptides were acetylated, while the C-termini were amidated.
[0570]Purification of cross-linked compounds was achieved by high performance liquid chromatography (HPLC) (Varian ProStar) on a reverse phase C18 column (Varian) to yield the pure compounds. Chemical composition of the pure products was confirmed by LC/MS mass spectrometry (Micromass LCT interfaced with Agilent 1100 HPLC system) and amino acid analysis (Applied Biosystems, model 420A).
Example 2: Metabolism by Purified Protease
[0571]Linear peptides and cross-linked peptidomimetic macrocycles are tested for stability to proteolysis by Trypsin (MP Biomedicals, Solon OH) by solubilizing each peptide at 10 μM concentration in 200 μL 100 mM NH4OAc (pH 7.5). The reaction is initiated by adding 3.5 μl of Trypsin (12.5 μg protease per 500 μL reaction) and shaking continually in sealed vials while incubating in a Room Temperature (22±2° C.). The enzyme/substrate ratio is 1:102 (w/w). After incubation times of 0, 5, 30, 60 and 135 min the reaction is stopped by addition of equal volume of 0.2% trifluoroacetic acid. Then, the solution is immediately analyzed by LC-MS in positive detection mode. The reaction half-life for each peptide is calculated in GraphPad Prism by a non-linear fit of uncalibrated MS response versus enzyme incubation time.
[0000] |
| 1628 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | E | F | N | $ | Y | Y | A | R | R | —NH2 | 1344.74 | 1345.7 | | 10.6 | 3.9 | 12.9 |
|
| 1629 | Ac- | | I | W | I | A | Q | E | L | R | $r8 | I | G | D | E | F | N | $ | Y | Y | A | R | R | —NH2 | 1373.75 | 1373.56 | | | 9.2 | 23.5 |
|
| 1630 | Ac- | | | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1103.1 | 1103.12 | | | 212.6 | 423.8 |
|
| 1631 | Ac- | | | | I | A | Q | A | L | R | $r8 | I | G | D | A | F | A | $ | Y | Y | A | | | —NH2 | 988.55 | 988.45 | | | 373.6 | 877.5 |
|
| 1632 | Ac- | | | | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | A | A | | | —NH2 | 964.04 | 963.94 | | | >1000 | >1000 |
|
| 1633 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1159.64 | 1159.87 | 6.6 | 8.4 | 22.4 | 84.8 |
|
| 1634 | Ac- | | | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1103.1 | 1102.94 | | | | 410.2 |
|
| 1635 | Ac- | | | | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1010.06 | 1009.9 | | | 308.6 | 519.2 |
|
| 1636 | Ac- | | | | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 981.55 | 981.86 | | | 255.9 | 318.7 |
|
| 1637 | Ac- | | | | I | A | Q | A | L | A | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 967.53 | 967.45 | | | >1000 | >1000 |
|
| 1638 | Ac- | | | | I | A | Q | A | L | R | $r8 | I | A | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1017.07 | 1016.93 | | | 243.1 | 272.5 |
|
| 1639 | Ac- | | | | I | A | Q | A | L | R | $r8 | I | G | D | A | A | N | $ | Y | Y | A | | | —NH2 | 972.04 | 971.89 | | | >1000 | >1000 |
|
| 1640 | Ac- | | | | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | A | Y | A | | | —NH2 | 964.04 | 963.94 | | | 471.5 | 803.9 |
|
| 1641 | Ac- | | I | $ | I | A | Q | $ | L | R | $r8 | I | G | D | E | F | N | $ | Y | Y | A | | | —NH2 | 1185.17 | 1185.61 | >40 | 19.5 | 11.6 | 8.7 |
|
| 1642 | Ac- | | I | W | I | A | Q | A | L | R | % r8 | I | G | D | A | F | N | % | Y | Y | A | | | —NH2 | 1160.14 | 1161.28 |
|
| 1643 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | E | F | A | $ | Y | Y | A | | | —NH2 | 1167.14 | 1168.2 | | 7.0 | 15.4 | 21.9 |
|
| 1644 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | Q | A | N | $ | Y | Y | A | | | —NH2 | 1150.13 | 1151.09 |
|
| 1645 | FITC- | Ba | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1368.67 | 1369.79 | | ND | ND | ND |
|
| 1646 | 5- | Ba | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1353.18 | 1354.13 | | ND | ND | ND |
| FAM- |
|
| 1647 | 5- | Ba | I | W | I | A | Q | A | L | R | $r8 | I | G | D | E | F | N | $ | Y | Y | A | | | —NH2 | 1382.18 | 1382.99 | | ND | ND | ND |
| FAM- |
|
| 1648 | Ac- | | I | A | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1102.12 | 1103.17 | | 19.7 | 22.3 | 37.7 |
|
| 1649 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | E | F | N | $ | Y | Y | A | | | —NH2 | 1188.64 | 1189.57 | >40 | 1.8 | 1.4 | 3.2 |
|
| 1650 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | Q | F | N | $ | Y | Y | A | | | —NH2 | 1188.15 | 1189.1 | | 5.2 | 12.0 | 67.0 |
|
| 1651 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | E | F | N | $ | Y | Y | A | | | —NH2 | 1160.13 | 1161.17 | | 1.0 | 1.0 | 6.0 |
|
| 1652 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | Q | F | N | $ | Y | Y | A | | | —NH2 | 1159.64 | 1160.34 | | 6.0 | 4.0 | 22.0 |
|
| 1653 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1131.13 | 1132.12 | | 6.7 | 25.6 | 65.4 |
|
| 1654 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | A | $ | Y | Y | A | | | —NH2 | 1138.14 | 1139.15 | | 7.4 | 55.7 | 114.6 |
|
| 1655 | Ac- | | I | W | I | A | Q | A | L | Cit | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1160.13 | 1160.98 | 9.1 | 7.5 | 109.0 | 211.6 |
|
| 1656 | Ac- | | I | W | I | A | Q | A | L | Cit | $r8 | I | G | D | Q | F | N | $ | Y | Y | A | | | —NH2 | 1188.64 | 1189.66 | | 1.7 | 28.8 | 88.2 |
|
| 1657 | Ac- | | I | W | I | A | Q | A | L | H | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1150.12 | 1151.09 | | >1000 | >1000 | >1000 |
|
| 1658 | Ac- | | I | W | I | A | Q | A | L | H | $r8 | I | G | D | Q | F | N | $ | Y | Y | A | | | —NH2 | 1178.63 | 1179.67 | | >1000 | >1000 | >1000 |
|
| 1659 | Ac- | | I | W | I | A | Q | A | L | Q | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1145.62 | 1146.55 | | 76.2 | 325.4 | 364.7 |
|
| 1660 | Ac- | | I | W | I | A | Q | A | L | Q | $r8 | I | G | D | Q | F | N | $ | Y | Y | A | | | —NH2 | 1174.13 | 1175.14 | | 14.8 | 6.3 | 27.5 |
|
| 1661 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | A | N | $ | Y | Y | A | | | —NH2 | 1121.62 | 1122.5 | | 7.5 | 401.7 | 139.7 |
|
| 1662 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | I | N | $ | Y | Y | A | | | —NH2 | 1142.65 | 1143.59 | | 3.4 | 14.1 | 113.0 |
|
| 1663 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | Q | I | N | $ | Y | Y | A | | | —NH2 | 1171.16 | 1171.9 |
|
| 1664 | Ac- | | I | W | I | A | Q | A | A | R | $r8 | I | G | D | A | A | N | $ | Y | Y | A | | | —NH2 | 1100.6 | 1101.5 | | 177.0 | 154.0 | 502.0 |
|
| 1665 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | A | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.83 | | 96.3 | 7.7 | 84.0 |
|
| 1666 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | A | D | Q | F | N | $ | Y | Y | A | | | —NH2 | 1195.16 | 1196.23 | | 116.2 | 7.7 | 25.6 |
|
| 1667 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | A | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1138.62 | 1139.61 | | 182.7 | 18.1 | 59.6 |
|
| 1668 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | A | G | D | Q | F | N | $ | Y | Y | A | | | —NH2 | 1167.13 | 1168.11 | | 122.1 | 1.9 | 4.8 |
|
| 1669 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | F | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1176.63 | 1177.63 | | 27.8 | 15.8 | 68.5 |
|
| 1670 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | F | G | D | Q | F | N | $ | Y | Y | A | | | —NH2 | 1205.14 | 1205.94 | | 74.1 | 25.6 | 66.1 |
|
| 1671 | Ac- | | I | W | F | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1176.63 | 1177.63 | | 22.0 | 28.0 | 179.4 |
|
| 1672 | Ac- | | I | W | F | A | Q | A | L | R | $r8 | I | G | D | Q | F | N | $ | Y | Y | A | | | —NH2 | 1205.14 | 1206.13 | | 29.3 | 25.9 | 204.6 |
|
| 1673 | Ac- | | I | W | I | A | Q | A | L | A | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1117.11 | 1118.15 | | 73.8 | 386.4 | >1000 |
|
| 1674 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | N | A | F | N | $ | Y | Y | A | | | —NH2 | 1159.15 | 1159.63 | | 194.7 | 416.0 | 404.9 |
|
| 1675 | Ac- | | I | W | I | A | Q | A | A | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1138.62 | 1139.2 | | >1000 | >1000 | >1000 |
|
| 1676 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | Q | F | A | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.3 | | 22.8 | 53.5 | 84.9 |
|
| 1677 | Ac- | | I | W | Cha | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1179.65 | 1180.15 | 3.9 | 43.8 | 14.4 | 104.9 |
|
| 1678 | Ac- | | I | W | hhL | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1173.65 | 1174.39 | 5.7 | 21.2 | 11.9 | 160.7 |
|
| 1679 | Ac- | | I | W | Adm | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1198.66 | 1199.28 | | 21.6 | 7.3 | 59.0 |
|
| 1680 | Ac- | | I | W | hCha | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1186.66 | 1186.98 | | 22.2 | 13.1 | 182.3 |
|
| 1681 | Ac- | | I | W | hF | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1183.64 | 1184.48 | 7.2 | 53.1 | 69.7 | 221.2 |
|
| 1682 | Ac- | | I | W | Igl | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1190.65 | 1190.41 | 5.9 | 12.8 | 145.5 | 246.4 |
|
| 1683 | Ac- | | I | W | F4CF3 | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1210.62 | 1211.31 | | 76.7 | 9.1 | 237.0 |
|
| 1684 | Ac- | | I | W | F4tBu | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1204.66 | 1205.39 | | 150.8 | 16.9 | >1000 |
|
| 1685 | Ac- | | I | W | 2Nal | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1201.64 | 1202.2 | 4.8 | 163.2 | 151.1 | 264.6 |
|
| 1686 | Ac- | | I | W | Bip | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1214.65 | 1215.43 | 6.4 | 11.0 | 3.0 | >1000 |
|
| 1687 | Ac- | | I | W | I | A | Q | A | Cha | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1179.65 | 1180.22 | | 4.2 | 81.1 | >1000 |
|
| 1688 | Ac- | | I | W | I | A | Q | A | hhL | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1173.65 | 1174.4 | | 3.1 | 135.9 | 231.4 |
|
| 1689 | Ac- | | I | W | I | A | Q | A | Adm | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1198.66 | 1199.05 | 0.5 | 40.2 | 109.5 | >1000 |
|
| 1690 | Ac- | | I | W | I | A | Q | A | hCha | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1186.66 | 1187.25 | | 3.8 | >1000 | >1000 |
|
| 1691 | Ac- | | I | W | I | A | Q | A | hAdm | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1205.67 | 1206.4 | | 16.6 | >1000 | 240.3 |
|
| 1692 | Ac- | | I | W | I | A | Q | A | hF | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1183.64 | 1184.29 | | 7.5 | >1000 | >1000 |
|
| 1693 | Ac- | | I | W | I | A | Q | A | Igl | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1190.65 | 1190.4 | | 47.7 | 146.7 | >1000 |
|
| 1694 | Ac- | | I | W | I | A | Q | A | F4CF3 | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1210.62 | 1210.94 | | 188.1 | 10.8 | >1000 |
|
| 1695 | Ac- | | I | W | I | A | Q | A | F4tBu | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1204.66 | 1205.29 | | 169.0 | 12.7 | 288.0 |
|
| 1696 | Ac- | | I | W | I | A | Q | A | 2Nal | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1201.64 | 1202.15 | | 119.7 | 17.3 | 234.4 |
|
| 1697 | Ac- | | I | W | I | A | Q | A | Bip | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1214.65 | 1214.91 | | 83.7 | 8.0 | 280.1 |
|
| 1698 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | Cba | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1165.64 | 1166.07 | | 26.6 | 27.5 | 89.0 |
|
| 1699 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | hL | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.37 | | 13.0 | 6.0 | 12.7 |
|
| 1700 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | Cha | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1179.65 | 1180.22 | | 15.9 | 7.1 | 109.1 |
|
| 1701 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | Tba | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.18 | | 13.7 | 35.4 | 227.1 |
|
| 1702 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | hhL | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1173.65 | 1173.93 | | 34.6 | 4.0 | 23.1 |
|
| 1703 | Ac- | | I | AmW | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.18 | | 9.9 | 17.4 | 70.6 |
|
| 1704 | Ac- | | I | Aib | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1109.13 | 1109.46 | | 42.5 | 83.5 | 97.9 |
|
| 1705 | Ac- | | AmL | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.27 | | 5.2 | 8.4 | 48.3 |
|
| 1706 | Ac- | | I | W | AmL | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1137.37 | | 19.8 | 7.2 | 24.8 |
|
| 1707 | Ac- | | I | W | I | Aib | Q | A | L | R | $r8 | I | G | AmD | A | F | N | $ | Y | Y | A | | | —NH2 | 1173.65 | 1173.93 | | >1000 | >1000 | >1000 |
|
| 1708 | Ac- | | I | W | I | A | Aib | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1138.14 | 1138.32 | | 5.5 | 59.0 | 120.1 |
|
| 1709 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | AmD | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.37 | >40 | >1000 | 15.5 | >1000 |
|
| 1710 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | F4F | A | | | —NH2 | 1160.64 | 1161.45 | 2.1 | 4.8 | 9.5 | 91.8 |
|
| 1711 | Ac- | | I | W | Tba | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.37 | | 10.9 | 17.2 | 36.6 |
|
| 1712 | Ac- | | I | W | hL | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.37 | | 3.7 | 17.0 | 36.5 |
|
| 1713 | Ac- | | I | W | Chg | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1172.65 | 1173.47 | | 4.6 | 20.9 | 38.9 |
|
| 1714 | Ac- | | I | W | Ac6c | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1165.64 | 1166.44 | | 10.4 | 7.7 | 25.7 |
|
| 1715 | Ac- | | I | W | Ac5c | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1158.63 | 1159.32 | | 8.9 | 8.4 | 68.2 |
|
| 1716 | Ac- | | E | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1139.11 | 1139.52 | | 2.2 | 72.0 | 117.8 |
|
| 1717 | Ac- | | R | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1152.64 | 1153.49 | | 4.5 | 32.8 | 47.8 |
|
| 1718 | Ac- | | K | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1138.63 | 1138.97 | | 3.9 | 27.2 | 49.7 |
|
| 1719 | Ac- | | H | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1143.12 | 1143.87 | | 3.6 | 25.2 | 52.0 |
|
| 1720 | Ac- | | S | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1118.1 | 1118.8 | | 3.9 | 33.4 | 53.2 |
|
| 1721 | Ac- | | Q | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1138.62 | 1139.24 | | 4.8 | 35.9 | 64.9 |
|
| 1722 | Ac- | | A | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1110.1 | 1110.75 | | 3.8 | 32.6 | 63.9 |
|
| 1723 | Ac- | | Aib | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1117.11 | 1117.78 | | 4.0 | 20.3 | 56.0 |
|
| 1724 | Ac- | | F | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1148.12 | 1148.96 | | 6.2 | 33.9 | 76.7 |
|
| 1725 | Ac- | | I | D | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1095.6 | 1096.32 | | 3.0 | 36.3 | 41.1 |
|
| 1726 | Ac- | | I | R | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1116.14 | 1116.95 | | 9.8 | 20.5 | 39.1 |
|
| 1727 | Ac- | | I | H | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1106.62 | 1107.24 | | 6.6 | 19.5 | 43.0 |
|
| 1728 | Ac- | | I | S | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1081.6 | 1181.98 | | 15.3 | 56.2 | 89.5 |
|
| 1729 | Ac- | | I | N | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1095.11 | 1095.58 | | 11.2 | 37.3 | 62.5 |
|
| 1730 | Ac- | | I | L | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1094.63 | 1095.3 | | 10.2 | 71.8 | 125.6 |
|
| 1731 | Ac- | | I | F | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1111.62 | 1112.33 | | 10.2 | 45.3 | 95.9 |
|
| 1732 | Ac- | | I | 2Nal | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1136.63 | 1137.3 | | 13.7 | 55.3 | 144.3 |
|
| 1733 | Ac- | | I | W | I | S | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1139.13 | 1139.89 | | 3.6 | 67.8 | 117.2 |
|
| 1734 | Ac- | | I | W | I | L | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1152.15 | 1152.94 | | 19.7 | 96.2 | 170.5 |
|
| 1735 | Ac- | | I | W | I | F | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1169.14 | 1169.86 | | 17.2 | 109.9 | 125.0 |
|
| 1736 | Ac- | | I | W | I | A | L | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1152.15 | 1152.84 | | 11.6 | 37.9 | 75.8 |
|
| 1737 | Ac- | | I | W | I | A | A | A | L | K | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1117.13 | 1117.97 | | 23.2 | 11.7 | 25.6 |
|
| 1738 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | Abu | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1145.14 | 1145.9 | | 106.2 | 112.2 | 130.6 |
|
| 1739 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | V | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1152.15 | 1152.94 | | 104.3 | 139.5 | 119.8 |
|
| 1740 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | E | A | F | N | $ | Y | Y | A | | | —NH2 | 1138.14 | 1138.87 | | 63.6 | 135.4 | 141.9 |
|
| 1741 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | G | N | $ | Y | Y | A | | | —NH2 | 1086.1 | 1086.89 | | 29.7 | 171.4 | 145.1 |
|
| 1742 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | W | N | $ | Y | Y | A | | | —NH2 | 1179.14 | 1180.04 | | 2.3 | 14.5 | 17.7 |
|
| 1743 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | hF | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.46 | | 2.7 | 16.6 | 38.9 |
|
| 1744 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F4CF3 | N | $ | Y | Y | A | | | —NH2 | 1193.63 | 1194.38 | | 8.2 | 107.4 | 103.8 |
|
| 1745 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F4tBu | N | $ | Y | Y | A | | | —NH2 | 1187.67 | 1188.36 | | 21.2 | 154.1 | 158.3 |
|
| 1746 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | 2Nal | N | $ | Y | Y | A | | | —NH2 | 1184.65 | 1185.5 | | 4.4 | 19.1 | 35.1 |
|
| 1747 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | Bip | N | $ | Y | Y | A | | | —NH2 | 1197.65 | 1198.54 | | 6.5 | 100.2 | 113.5 |
|
| 1748 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | D | $ | Y | Y | A | | | —NH2 | 1131.62 | 1132.4 | | 1.5 | 25.9 | 35.3 |
|
| 1749 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | E | $ | Y | Y | A | | | —NH2 | 1138.63 | 1139.02 | | 1.8 | 17.9 | 30.7 |
|
| 1750 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | Q | $ | Y | Y | A | | | —NH2 | 1138.14 | 1138.84 | | 4.9 | 36.5 | 71.6 |
|
| 1751 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | S | $ | Y | Y | A | | | —NH2 | 1117.62 | 1118.5 | | 8.0 | 44.1 | 67.5 |
|
| 1752 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | H | $ | Y | Y | A | | | —NH2 | 1142.64 | 1143.25 | | 8.0 | 36.3 | 57.4 |
|
| 1753 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | L | Y | A | | | —NH2 | 1106.14 | 1107.05 | | 17.6 | 69.9 | 124.9 |
|
| 1754 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | A | A | | | —NH2 | 1113.63 | 1114.27 | | 20.3 | 51.8 | 102.0 |
|
| 1755 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | L | A | | | —NH2 | 1134.65 | 1135.33 | | 23.4 | 9.0 | 18.9 |
|
| 1756 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Cha | A | | | —NH2 | 1154.66 | 1155.31 | | 24.1 | 8.6 | 28.9 |
|
| 1757 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | hF | A | | | —NH2 | 1158.65 | 1159.5 | | 8.0 | 12.1 | 30.7 |
|
| 1758 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | W | A | | | —NH2 | 1171.15 | 1171.78 | | 3.9 | 15.4 | 23.5 |
|
| 1759 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | 2Nal | A | | | —NH2 | 1176.65 | 1177 | | 8.0 | 26.1 | 65.2 |
|
| 1760 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | D | | | —NH2 | 1153.12 | 1153.77 | | 2.2 | 116.4 | 137.9 |
|
| 1761 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | E | | | —NH2 | 1160.13 | 1160.8 | | 1.4 | 45.4 | 56.4 |
|
| 1762 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | Q | | | —NH2 | 1159.64 | 1160.26 | | 4.6 | 41.1 | 64.7 |
|
| 1763 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | S | | | —NH2 | 1139.13 | 1139.47 | | 4.7 | 36.0 | 62.4 |
|
| 1764 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | H | | | —NH2 | 1164.14 | 1165.05 | | 10.6 | 73.8 | 98.8 |
|
| 1765 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | R | | | —NH2 | 1173.66 | 1174.4 | | 18.5 | 185.9 | 141.8 |
|
| 1766 | Ac- | | I | W | I | A | A | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | K | | | —NH2 | 1159.66 | 1160.26 | | 6.6 | 66.3 | 43.4 |
|
| 1767 | Ac- | | I | W | I | A | Q | A | AmL | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.18 | 0.98 | 86.6 | >1000 | >1000 |
|
| 1768 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | AmD | A | F | N | $ | Y | Y | A | | | —NH2 | 1166.65 | 1167.46 | 15.2 | >1000 | 205.5 | >1000 |
|
| 1769 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | F4F | Y | A | | | —NH2 | 1160.64 | 1161.26 | 1.4 | 14.9 | 26.0 | 199.8 |
|
| 1770 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | Y | Y | Aib | | | —NH2 | 1166.65 | 1167.46 | 4.6 | 29.0 | >1000 | 218.1 |
|
| 1771 | Ac- | | I | W | I | A | Q | A | A | Cit | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | —NH2 | 1139.11 | 1139.71 | 15.3 | >1000 | 85.0 | >1000 |
|
| 1772 | Ac- | | I | W | I | A | Q | A | L | Cit | $r8 | I | G | N | A | F | N | $ | Y | Y | A | | | —NH2 | 1159.64 | 1160.4 | 5.0 | >1000 | >1000 | >1000 |
|
| 1773 | Ac- | | I | W | I | A | Q | A | L | Cit | $r8 | I | G | D | A | A | N | $ | Y | Y | A | | | —NH2 | 1122.12 | 1122.87 | 19.3 | 39.5 | >1000 | >1000 |
|
| 1774 | Ac- | | I | W | I | A | Q | A | L | Cit | $r8 | I | G | D | A | V | N | $ | Y | Y | A | | | —NH2 | 1136.13 | 1136.47 | 5.8 | 0.8 | >1000 | >1000 |
|
| 1775 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | F | N | $ | A | Y | A | | | —NH2 | 1113.63 | 1113.9 | 4.0 | 5.3 | 12.6 | 111.6 |
|
| 1776 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | hL | G | D | A | F | N | $ | F4F | Y | A | | | —NH2 | 1167.64 | 1168.57 | 1.0 | 58.0 | 43.0 |
|
| 1777 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | hL | G | D | A | F | N | $ | Y | F4F | A | | | —NH2 | 1167.64 | 1168.2 | 0.7 | 27.0 | 13.0 |
|
| 1778 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | hL | G | D | A | F | N | $ | F4F | F4F | A | | | —NH2 | 1168.64 | 1169.59 | 0.7 | 127.0 | 121.0 |
|
| 1779 | Ac- | | A | W | I | A | A | A | L | R | $r8 | hL | G | D | A | F | N | $ | Y | F4F | A | | | —NH2 | 1118.11 | 1118.89 | 0.6 | 52.0 | 37.0 |
|
| 1780 | Ac- | | A | W | I | A | A | A | L | R | $r8 | hL | G | D | A | F | N | $ | A | F4F | A | | | —NH2 | 1072.1 | 1072.92 | 0.9 | 23.0 | 9.0 |
|
| 1781 | Ac- | | I | W | I | A | Q | A | A | R | $r8 | hL | G | D | A | F | N | $ | F4F | F4F | A | | | —NH2 | 1147.62 | 1148.59 | 0.5 | >1000 | >1000 |
|
| 1782 | Ac- | | I | $r8 | I | A | Q | A | L | R | St | I | G | D | E | F | N | $s8 | Y | Y | A | | | —NH2 | 1199.18 | 1199.74 | >40 | 1.1 | 1.1 | 22.0 |
|
| 1783 | Ac- | | I | W | I | A | $ | A | L | R | St | I | G | D | E | F | N | $s8 | Y | Y | A | | | —NH2 | 1207.17 | 1207.7 | >40 | 1.6 | 1.6 | 19.2 |
|
| 1784 | Ac- | | I | W | I | A | Q | A | L | R | $r8 | I | G | D | E | F | N | St | Y | Y | A | $r5 | A | —NH2 | 1306.72 | 1307.42 | >40 | 11.6 | 24.2 | 57.7 |
|
| *Raji Cell Viability, 48 h, 5% serum |
Example 3: Dose-Dependent Cell Killing by Peptidomimetic Macrocycles
[0572]Aileron peptide A is formulated as a pharmaceutical formulation. Aileron peptide A is an alpha helical hydrocarbon cross-linked polypeptide macrocycle, with an amino acid sequence less than 25 amino acids long that is derived from BCL-2-like protein 11 (BIM). Aileron peptide A has a single cross link spanning amino acids in the i to the i+4 position of the amino acid sequence and has 8 amino acids between the i+4 position and the carboxyl terminus. Aileron peptide A binds to MCL-1 or a BCL-2 family protein to trigger apoptosis, and has a molecular weight in the range of 2500-2550 m/e.
[0573]Aileron peptide B is formulated as a pharmaceutical formulation. Aileron peptide B is an alpha helical hydrocarbon cross-linked polypeptide macrocycle, with an amino acid sequence less than 20 amino acids long that is derived from BCL-2-like protein 11 (BIM). Aileron peptide B has a single cross link spanning amino acids in the i to the i+7 position of the amino acid sequence and has 8 amino acids between the i+7 position and the carboxyl terminus. Aileron peptide 1 binds to MCL-1 or a BCL-2 family protein to trigger apoptosis, and has a molecular weight in the range of 2250-2300 m/e.
[0574]Aileron peptide C is formulated as a pharmaceutical formulation. Aileron peptide C is an alpha helical hydrocarbon cross-linked polypeptide macrocycle, with an amino acid sequence less than 25 amino acids long that is derived from BCL-2-like protein 11 (BIM). Aileron peptide C has a single cross link spanning amino acids in the i to the i+7 position of the amino acid sequence and has 3 amino acids between the i+7 position and the carboxyl terminus. Aileron peptide C binds to MCL-1 or a BCL-2 family protein to trigger apoptosis, and has a molecular weight in the range of 2500-2600 m/e.
[0575]BIM peptidomimetic macrocycles were tested for cell killing at various concentrations. Human Raji cells were treated with increasing doses of peptidomimetic macrocycles corresponding to Aileron peptide A (FIGS. 1 and 2), Aileron peptide B (FIGS. 1-3), and Aileron peptide C (FIGS. 3 and 4). An % Viable cells was calculated for each dose of the peptidomimetic macrocycle from a non-linear fit of response vs dose (GraphPad Prism). The effect of the peptidomimetic macrocycles corresponding to Aileron peptide A are presented in FIGS. 1 and 2. The effect of the peptidomimetic macrocycles corresponding to Aileron peptide B are presented in FIGS. 1-3. The effect of the peptidomimetic macrocycles corresponding to Aileron peptide C are presented in FIGS. 3 and 4.
Example 4: MCL-1 Displacement Study
[0576]BIM peptidomimetic macrocycles were tested for displacement of MCL-1 from a BAK fluorescence resonance energy transfer (FRET) peptide. Human Raji cells were treated with DMSO, ABT-263, and peptidomimetic macrocycles corresponding to Aileron peptide A and Aileron peptide B. FIG. 5 shows the effect of the compounds on normalized BAK peptide FRET signal.
Example 5: Pharmacokinetic (PK) and Bio-Distribution Study in Mice
[0577]A peptidomimetic macrocycle corresponding to Aileron peptide A was administered to mice at a 5 mg/kg dose. Mice were sacrificed at specific time points both before and after dosing, up to 24 hours post-administration. Blood, liver, and spleen were collected from the mice at the specific time points. Plasma was prepared from the blood using K2EDTA tubes by centrifuging for 20 minutes at 4° C. at 2000G maximum 30 minutes after collection. From each plasma sample, an aliquot was transferred to a fresh tube for PK studies. From each liver and spleen sample, tissue was homogenized and extracts were prepared for bio-distribution studies. FIG. 6 shows the PK and bio-distribution results for this study by concentration in nanograms of peptidomimetic macrocycle per gram mouse body weight (ng/g) over time.
Example 6: Human Plasma Stability Study
[0578]Peptidomimetic macrocycles corresponding to Aileron peptide A or Aileron peptide B were administered to humans. Blood was collected at specific time points both before and after dosing, up to 24 hours post-administration. Plasma was prepared from the blood using K2EDTA tubes by centrifuging for 20 minutes at 4° C. at 2000G maximum 30 minutes after collection. From each plasma sample, an aliquot was transferred to a fresh tube for plasma stability studies. FIG. 7 shows the plasma stability results for this study as a percentage of peptidomimetic macrocycle remaining in plasma over time, with the dashed line corresponding to the initial amount of peptidomimetic macrocycle dosed.
Example 7: Cell Viability and Caspase-3/7 Assay
[0579]Cancer cells were cultured using a standard culture medium containing 10% fetal bovine serum (FBS) and penicillin-streptomycin (A375P: DMEM; SK-MEL-2, SK-MEL-28: EMEM). Cells were plated in 96-well plates (5×103 cells per well) and, after overnight incubation, treated with the indicated concentrations of Stapled Peptides in the corresponding medium supplemented with 5% FBS for the indicated durations. Cell viability and caspase-3/7 activation was measured using CellTiter-Glo and Caspase-Glo 3/7 chemiluminescence reagents (Promega), respectively. Luminescence was detected by a microplate reader (Spectramax M5, Molecular Devices).
[0580]Aileron peptide 1 is formulated as a pharmaceutical formulation. Aileron peptide 1 is a warhead-containing alpha helical hydrocarbon cross-linked polypeptide macrocycle, with an amino acid sequence less than 25 amino acids long that is derived from BCL-2-like protein 11 (BIM). Aileron peptide 1 has a single cross link spanning amino acids in the i to the i+4 position of the amino acid sequence and has 8 amino acids between the i+4 position and the carboxyl terminus. Aileron peptide 1 binds to BFL-1 or a BCL-2 family protein to trigger apoptosis, and has a molecular weight in the range of 2500-2600 m/e.
[0581]Aileron peptide 2 is formulated as a pharmaceutical formulation. Aileron peptide 2 is an alpha helical hydrocarbon cross-linked polypeptide macrocycle, with an amino acid sequence less than 20 amino acids long that is derived from BCL-2-like protein 11 (BIM). Aileron peptide 2 has a single cross link spanning amino acids in the i to the i+7 position of the amino acid sequence and has 3 amino acids between the i+7 position and the carboxyl terminus. Aileron peptide 2 binds to BFL-1 or a BCL-2 family protein to trigger apoptosis, and has a molecular weight in the range of 2500-2600 m/e.
[0582]Aileron peptide 3 is formulated as a pharmaceutical formulation. Aileron peptide 3 is a warhead-containing alpha helical hydrocarbon cross-linked polypeptide macrocycle, with an amino acid sequence less than 20 amino acids long that is derived from BCL-2-like protein 11 (BIM). Aileron peptide 3 has a single cross link spanning amino acids in the i to the i+7 position of the amino acid sequence and has 3 amino acids between the i+7 position and the carboxyl terminus. Aileron peptide 3 binds to BFL-1 or a BCL-2 family protein to trigger apoptosis, and has a molecular weight in the range of 2400-2500 m/e.
[0583]FIG. 8 shows the results of treating A375P-cells with BIM SAHBA1and Aileron peptide 1 (40 μM). The results show that neither BIM SAHBA1nor Aileron peptide 1 affected proliferation and apoptosis induction in A375-P melanoma cells.
[0584]FIG. 9 shows the results of treating SK-MEL-2 cells with BIM SAHBA1and Aileron peptide 1 (40 μM). The results show that neither BIM SAHBA1nor Aileron peptide 1 affected proliferation and apoptosis induction in SK-MEL-2 melanoma cells.
[0585]FIG. 10 shows the results of treating SK-MEL-28 cells with BIM SAHBA1and Aileron peptide 1 (40 μM). The results show that neither BIM SAHBA1nor Aileron peptide 1 affected proliferation and apoptosis induction in SK-MEL-28 melanoma cells.
[0586]FIG. 11 shows the results of treating A375-P cells with Aileron peptide 2 or Aileron peptide 3 (40 μM). The results show that Aileron peptide 2 and Aileron peptide 3 inhibited proliferation and induced apoptosis in A375-P cells.
[0587]FIG. 12 shows the results of treating SK-MEL-2 cells with Aileron peptide 2 or Aileron peptide 3 (40 μM). The results show that Aileron peptide 2 and Aileron peptide 3 inhibited proliferation and induced apoptosis in SK-MEL-2 cells.
[0588]FIG. 13 shows the results of treating SK-MEL-28 cells with Aileron peptide 2 or Aileron peptide 3 (40 μM). The results show that Aileron peptide 2 and Aileron peptide 3 inhibited proliferation and induced apoptosis in SK-MEL-28 cells.
Example 8: Mechanism of Action of Stapled BIM Peptides
[0589]The stapled BIM peptides of the disclosure can inhibit anti-apoptotic proteins, including BCL-2, MCL-1, and BCL-XL. The stapled BIM peptides of the disclosure can also directly active BAX/BAK, which are two nuclear-encoded proteins present in higher eukaryotes that are able to pierce the mitochondrial outer membrane to mediate cell death by apoptosis. Organelles recruited by nucleated cells to supply energy that can be recruited by BAX and BAK to kill cells. The two proteins lie in wait in healthy cells, where they adopt a globular α-helical structure as monomers.
[0590]Following a variety of stress signals, BAX and BAK convert into pore-forming proteins by changing conformations and assembling into oligomeric complexes in the mitochondrial outer membrane. Proteins from the mitochondrial intermembrane space and empty into the cytosol to activate proteases that dismantle the cell. FIG. 14 illustrates how a stapled peptide derived from the protein BIM broadly targets BCL-2 family proteins, neutralizes BIM's prosurvival relatives (e.g., BCL-2, MCL-1, and BCLXL), and directly activates BAX. FIG. 15 illustrates how a BH3-only protein (BIM) can directly activate mitochondrial BAK and cytosolic BAX, and inhibit the capacity of anti-apoptotic proteins to sequester activated forms of BAK and BAX, leading the inactive monomers of BAK and BAX to transform to toxic pore-forming proteins.
Example 9: Crystal Structure of Stapled BIM Peptide Bound to MCL-1
[0591]FIG. 16 compares high resolution X-ray structures of: a stapled BIM peptide bound to MCL-1; Noxa BH3 bound to MCL-1 (Peptide: PDB: 2NLA); and BIM BH3 bound to MCL-1 (Peptide: PDB: 2NL9). FIG. 17 shows a 2 angstrom X-ray structure of a stapled BIM-BH3 peptide bound to MCL-1. The X-ray crystal structure showed that the crosslinker of the peptide was a cis-olefin.
Example 10: Evaluation of the Biological Activity of Stapled BIM Peptides
[0592]The sequence information for the cross-linked peptides used in the studies are shown in TABLE 5. Aib represents 2-aminoisobutyric acid. $ represents an alpha-Me S5-pentenyl-alanine olefin amino acid connected to another amino acid side chain by an all-carbon crosslinker comprising one double bond, and $r8 represents an alpha-Me R8-octenyl-alanine olefin amino acid connected to another amino acid side chain by an all-carbon crosslinker comprising one double bond.
[0000] | |
| SEQUENCE | SEQ ID |
| # | | 146 | 147 | 148 | 149 | 150 | 151 | 152 | 153 | 154 | 155 | 156 | 157 | 158 | 159 | 160 | 161 | 162 | 163 | 164 | | | | NO: |
|
| 1 | Ac | I | W | I | A | Q | A | L | R | $r8 | I | G | D | E | F | N | $ | Y | Y | A | | | NH2 | 1333 |
|
| 2 | Ac | I | W | I | A | Q | A | L | R | $r8 | I | G | D | Q | F | N | $ | Y | Y | A | | | NH2 | 1334 |
|
| 3 | Ac | I | W | I | A | A | A | L | R | $r8 | I | G | D | E | F | N | $ | Y | Y | A | | | NH2 | 1335 |
|
| 4 | Ac | I | W | I | A | A | A | L | R | $r8 | I | G | D | Q | F | N | $ | Y | Y | A | | | NH2 | 1336 |
|
| 5 | Ac | I | W | I | A | Q | A | L | Cit | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | NH2 | 1341 |
|
| 6 | Ac | I | W | I | A | Q | A | L | Cit | $r8 | I | G | D | Q | F | N | $ | Y | Y | A | | | NH2 | 1342 |
|
| 7 | Ac | I | W | I | A | Q | A | L | R | $r8 | I | G | D | A | A | N | $ | Y | Y | A | | | NH2 | 1347 |
|
| 8 | Ac | I | W | I | A | Q | A | L | R | $r8 | I | G | D | Q | A | N | $ | Y | Y | A | | | NH2 | 1348 |
|
| 9 | Ac | I | W | I | A | Q | A | L | R | $r8 | I | A | D | Q | F | N | $ | Y | Y | A | | | NH2 | 1353 |
|
| 10 | Ac | I | W | I | A | Q | A | L | R | $r8 | A | G | D | Q | F | N | $ | Y | Y | A | | | NH2 | 1355 |
|
| 11 | Ac | I | W | I | A | Q | A | L | A | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | NH2 | 1361 |
|
| 12 | Ac | I | W | I | A | Q | A | L | R | $r8 | I | G | N | A | F | N | $ | Y | Y | A | | | NH2 | 1362 |
|
| 13 | Ac | I | W | I | A | Q | A | A | R | $r8 | I | G | D | A | F | N | $ | Y | Y | A | | | NH2 | 1363 |
|
| 14 | Ac | R | W | I | A | Q | A | L | R | $ | I | G | D | $ | L | N | Aib | F | Y | A | H | H | NH2 | 763 |
|
| 15 | Ac | I | W | I | A | Q | A | L | R | $r8 | I | G | D | E | F | N | $ | Y | Y | A | R | R | NH2 | 545 |
|
| 16 | Ac | I | W | I | A | Q | A | L | R | $r8 | hL | g | D | A | F | N | $ | Y | F4F | A | | | NH2 | 1621 |
|
[0593]The binding spectrum of stapled BIM BH3 peptides were tuned for BCL-2 family selectivity. TABLE 6 shows the Ki values (nM) of MCL-1, BCL-xL, and BCL-2 for ABT-199, and peptide #1-peptide #13. ABT-199 is venetoclax, and † represents values reported in the literature.
[0000] |
| ABT-199† | >444 | 48 | <0.01 | Bcl-2 selective |
| 1 | 1.8 | 1.4 | 3.2 | Pan-selective |
| 2 | 5.2 | 12 | 67 | Pan-selective |
| 3 | 1 | 1 | 6 | Pan-selective |
| 4 | 6 | 4 | 22 | Pan-selective |
| 5 | 7.5 | 109.9 | 211.6 | Mcl-1 selective |
| 6 | 1.7 | 28.8 | 88.2 | Mcl-1 selective |
| 7 | 7.5 | 401.7 | 139.7 | Mcl-1 selective |
| 8 | 1.4 | 24.9 | 43.9 | Mcl-1 selective |
| 9 | 116.2 | 7.7 | 25.6 | Bcl-xL/Bcl-2 selective |
| 10 | 122.1 | 1.9 | 4.8 | Bcl-xL/Bcl-2 selective |
| 11 | 73.8 | 386.4 | 1094.8 | negative control |
| 12 | 194.7 | 416.0 | 404.9 | negative control |
| 13 | 500.7 | 100000 | 100000 | negative control |
|
[0594]The stapled BIM peptides were shown to disrupt the formation of MCL-1/BAK complexes in living cells. FIG. 18 illustrates how stapled BIM peptides of the disclosure can disrupt the formation of MCL-1/BAK complexes in living cells. An assay was performed to determine the inhibitory constant (Ki) of BCL-xL, BCL-2, and MCL-1 in the presence of cross-linked peptide #14. The data show that in the presence of cross-linked peptide #14, the Kiof MCL-1 was drastically lower than the Kiof BCL-xLor BCL-2. TABLE 7 shows the results of the assay.
[0000] |
| BCL-xLKi(nM) | 178 |
| BCL-2 Ki(nM) | 151 |
| MCL-1 Ki(nM) | 11 |
| |
[0595]FIG. 19 compares normalized FRET signals of samples to determine the samples' effects in disrupting MCL-1/BAK protein-protein interactions. Cross-linked peptide #14 was highly effective in disrupting the MCL-1/BAK protein-protein interaction at concentrations of 10 μM and 20 μM. Cross-linked peptide #14 was equally effective at disrupting the interaction of MCL-1/BAK at 10 μM and 20 μM. ABT-263 (navitoclax) did not disrupt the protein-protein interaction of MCL-1/BAK. ABT-263 did not disrupt the protein-protein interaction of MCL-1/BAK at concentrations of 5 μM or 10 μM.
[0596]Peptides #14, #15, and #16 were tested against BH3 mimetic ABT-737, ABT-263 (navitoclax), and ABT-199 (venetoclax). TABLE 8 shows that crosslinked-peptide #16 was the most effective BIM stapled peptide. † represents valued reported in the literature.
[0000]

[0000] |
| BIM-SAHBA1 | Pan-selective | 2.7 | 6.2 | 29.6 |
| Peptide #14 | Mcl-1 selective | 17 | 114.5 | 214.7 |
| Peptide #15 | Pan-selective | 10.6 | 5.2 | 12.8 |
| Peptide #16 | Pan-selective | 27 | 13 | ND |
| ABT-737 | Bcl-xL/Bcl-2 selective | >1000 | 1.7 | 3.1 |
| ABT-263 | Bcl-xL/Bcl-2 selective | >1000 | 0.4 | 0.9 |
| ABT-263† | Bcl-xL/Bcl-2 selective | >224 | 0.055 | 0.044 |
| ABT-199† | Bcl-2 selective | >444 | 48 | <0.01 |
|
Lactate Dehydrogenase Cytotoxicity Colorimetric Assay
[0597]When cell membranes are compromised or damaged, lactate dehydrogenase (LDH), a soluble yet stable enzyme found inside every living cell, is released into the surrounding extracellular space. The presence of LDH in the culture medium can be used as a cell death marker. The relative amounts of live and dead cells within the medium can then be quantified by measuring the amount of released LDH using a colorimetric or fluorimetric LDH cytotoxicity assay. When using an LDH colorimetric assay, the amount of LDH released in the surrounding environment is measured with an enzymatic reaction that converts iodonitrotetrazolium (INT) into red-colored formazan. When LDH is present in the cell culture, the LDH reduces NAD+ to NADH and H+ through the oxidation of lactate to pyruvate. Afterward, the catalyst (diaphorase) then transfers H/H+ from NADH+H+ to the trazolium salt INT to form the red-colored formazan salt. The amount of color produced is measured at 490 nm by standard spectroscopy, and is proportional to the amount of damaged cells in the culture.
[0598]Cross-linked peptide #16 exhibited on-mechanism cytotoxic activity in BAX-BAK″wtMEF cells, but not BAX-BAK−/− double-knock outs. No off-target cytotoxicity was observed for peptide #16 in the LDH assay (all with 5% serum). FIG. 20 shows that cross-linked peptide #16 exhibited on-mechanism cytotoxic activity against BAX-BAKwt/wt(●) MEF cells but did not exhibit on-mechanism cytotoxic activity in BAX-BAK−/− double knock outs (DKO) (▴).
Apoptotic Response Against BFL-1-Drive Melanoma Cell Lines
[0599]Cross-linked peptide #16 was tested to determine the compound's ability to yield an enhanced apoptotic response against BFL-1-drive melanoma cell lines. Relative caspase-3/7 activation and % cell viability were measured using A375-P, SK-MEL-2, and SK-MEL-28 cell lines. BIM SAHBA1(40 μM, 5% serum) was used as a control. Consistent with greater cell potency, treatment of the cell lines with Peptide #16 induced higher levels of caspase-3/7 activation compared to the control. FIG. 21 shows that treatment of A375-P (1), SK-MEL-2 (2), and SK-MEL-28 (3) with peptide #16 induced higher levels of caspase-3/7 activation than the BIM SAHBA1control. FIG. 22 shows that treatment of A375-P (1), SK-MEL-2 (2), and SK-MEL-28 (3) with peptide #16 decreased the % viability of the cells, while treatment with BIM SAHBA1had no effect on % viability.
Anti-Proliferative Activity in ABT-199 Resistant Burkitt Lymphoma Raji Cell Line
[0600]WST-1 is a cell proliferation reagent that is used in colorimetric assays designed to measure the relative proliferation rates of cells in culture. The assay is based on the conversion of the tetrazolium salt WST-1 into a colored dye by mitochondrial dehydrogenase enzymes. The soluble salt is released into the media. Within a given time period, the reaction produces a color change that is directly proportional to the amount of mitochondrial dehydrogenase in a culture. The WST-1 assay measures the net metabolic activity of cells.
[0601]Raji cell proliferation was measured by treating ABT-199 resistant Burkitt lymphoma Raji cells with BIM SAHBA1, ABT-199, and Peptide #16. FIG. 23 shows that peptide #16 was ten times more potent than BIM SAHBA1in the MCL-1-1 driven Raji cell line. TABLE 9 shows the IC50 values calculated using the data presented in FIG. 22.
Anti-Proliferative Effects
[0602]Combination Treatment with Peptide #16 with ABT-199
[0603]Fixed doses of cross-linked peptide #16 were combined with varying levels of ABT-199 (venetoclax) to evaluate the anti-proliferative effects of combination treatment. Raji cell proliferation was determined by treating cells with ABT-199 (●); ABT-199+0.95 μM peptide #16 (▪); ABT-199+1.9 μM peptide #16 (▴); and ABT-199+3.8 μM peptide #16 (▾). The anti-proliferative effects of BCL-2-selective ABT-199 (EC503.7-4.9 μM) were enhanced by BIM-stapled peptide #16, a potent MCL-1 inhibitor, in MCL-1 driven Raji cells. FIG. 24 shows that Raji cell proliferation (fraction of control) decreased with increasing doses of peptide #16 in a dose-dependent manner.
[0604]Raji cell proliferation was also determined by treating cells with peptide #16 (●); peptide #16+1.9 μM ABT-199 (▪); peptide #16+3.8 μM ABT-199 (▴); and peptide #16+3.8 μM ABT-199 (▾). The anti-proliferative effects of BCL-2-selective peptide #16 (EC501.2-1.6 μM) were enhanced by ABT-199 in MCL-1 driven Raji cells. FIG. 25 shows that Raji cell proliferation (fraction of control) decreased with increasing doses of ABT-199 in a dose-dependent manner.
[0605]The ABT-199/Peptide #16 combination studies revealed additive to synergistic complementarity effects. FIG. 26 shows that the combination index (CI) of the combination study had additive to synergistic complementary effects.
EMBODIMENTS
[0606]The following non-limiting embodiments provide illustrative examples of the invention, but do not limit the scope of the invention.
Embodiment 1
[0607]A peptidomimetic macrocycle of Formula (Ic):
[0000]
[0608]each A, C, D, E, and F is independently a natural or non-natural amino acid;
[0609]each B is independently a natural or non-natural amino acid, amino acid analogue,
[0000]
[0000][—NH-L3-CO—], [—NH-L3-SO2—], or [—NH-L3-];
[0610]WH is an amino acid with an electron accepting group susceptible to attack by a nucleophile;
[0611]each L is independently a macrocycle-forming linker;
[0612]each L′ is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5, or a bond, or together with R1and the atom to which both R1and L′ are bound forms a ring;
[0613]each L″ is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene, each being optionally substituted with R5, or a bond, or together with R2and the atom to which both R2and L″ are bound forms a ring;
[0614]each R1is independently-H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, each being optionally substituted with halo-, or together with L′ and the atom to which both R1and L′ are bound forms a ring;
[0615]each R2is independently-H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, cycloalkylalkyl, heteroalkyl, or heterocycloalkyl, each being optionally substituted with halo-, or together with L″ and the atom to which both R2and L″ are bound forms a ring;
[0616]each R3is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, heteroalkyl, cycloalkyl, heterocycloalkyl, cycloalkylalkyl, aryl, or heteroaryl, each being optionally substituted with R5;
[0617]each L3is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, heteroarylene, or [—R4—K—R4-]n, each being optionally substituted with R5;
[0618]each R4is independently alkylene, alkenylene, alkynylene, heteroalkylene, cycloalkylene, heterocycloalkylene, arylene, or heteroarylene;
[0619]each K is independently O, S, SO, SO2, CO, CO2, or CONR3;
[0620]each n is independently 1, 2, 3, 4, or 5;
[0621]each R5is independently halogen, alkyl, —OR6, —N(R6)2, —SR6, —SOR6, —SO2R6, —CO2R6, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0622]each R6is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkylalkyl, heterocycloalkyl, a fluorescent moiety, a radioisotope, or a therapeutic agent;
[0623]each R7is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with a D residue;
[0624]each R8is independently —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, aryl, or heteroaryl, each being optionally substituted with R5, or part of a cyclic structure with an E residue;
[0625]each v and w is independently an integer from 1-1000;
[0627]u is 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10; and
[0628]each x, y and z is independently 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, or 10, or
[0000]a pharmaceutically-acceptable salt thereof.
Embodiment 2
[0629]The peptidomimetic macrocycle of embodiment 1, wherein the peptidomimetic macrocycle comprises two crosslinks, wherein a first crosslink is of a first pair of amino acid residues, and a second crosslink is of a second pair of amino acid residues.
Embodiment 3
[0630]The peptidomimetic macrocycle of embodiment 1 or 2, wherein the first pair of amino acid residues and the second pair of amino acid residues do not share a common amino acid residue.
Embodiment 4
[0631]The peptidomimetic macrocycle of embodiments 1 or 2, wherein the first pair of amino acid residues and the second pair of amino acid residues share one common amino acid residue.
Embodiment 5
[0632]The peptidomimetic macrocycle of any one of embodiments 1-4, wherein w is at least 2 and at least two E amino acids are His residues.
Embodiment 6
[0633]The peptidomimetic macrocycle of any one of embodiments 1-5, wherein the peptidomimetic macrocycle comprises a helix.
Embodiment 7
[0634]The peptidomimetic macrocycle of any one of embodiments 1-6, wherein the peptidomimetic macrocycle comprises an α-helix.
Embodiment 8
[0635]The peptidomimetic macrocycle of any one of embodiments 1-7, wherein each of v and w is independently 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, or 15.
Embodiment 9
[0636]The peptidomimetic macrocycle of any one of embodiments 1-8, wherein each of v and w is independently 3, 4, 5, 6, 7, 8, 9, or 10.
Embodiment 10
[0637]The peptidomimetic macrocycle of any one of embodiments 1-9, wherein v is 8.
Embodiment 11
[0638]The peptidomimetic macrocycle of any one of embodiments 1-10, wherein w is 6.
Embodiment 12
[0639]The peptidomimetic macrocycle of any one of embodiments 1-11, wherein L is
[0000]
Embodiment 13
[0640]The peptidomimetic macrocycle of any one of embodiments 1-12, wherein R1and R2are H.
Embodiment 14
[0641]The peptidomimetic macrocycle of any one of embodiments 1-12, wherein R1and R2are independently alkyl.
Embodiment 15
[0642]The peptidomimetic macrocycle of any one of embodiments 1-12 and 14, wherein R1and R2are methyl.
Embodiment 16
[0643]The peptidomimetic macrocycle of any one of embodiments 1-15, wherein the peptidomimetic macrocycle exhibits a selectivity ratio of one target over another that is from about 2:1 to about 1000:1.
Embodiment 17
[0644]The peptidomimetic macrocycle of any one of embodiments 1-16, wherein the peptidomimetic macrocycle exhibits a selectivity ratio of one target over another that is from about 5:1 to about 1000:1.
Embodiment 18
[0645]The peptidomimetic macrocycle of any one of embodiments 1-17, wherein the peptidomimetic macrocycle exhibits a selectivity ratio of one target over another that is from about 10:1 to about 1000:1.
Embodiment 19
[0646]The peptidomimetic macrocycle of any one of embodiments 1-18, wherein the peptidomimetic macrocycle exhibits a selectivity ratio of one target over another that is from about 100:1 to about 1000:1.
Embodiment 20
[0647]The peptidomimetic macrocycle of any one of embodiments 1-19, wherein the peptidomimetic macrocycle comprises an amino acid sequence that has at least 60% identity to any one of SEQ ID NOs.: 1-1625.
Embodiment 21
[0648]The peptidomimetic macrocycle of any one of embodiments 1-20, wherein the peptidomimetic macrocycle comprises an amino acid sequence that has at least 60% identity to any one of SEQ ID NOs.: 2-400.
Embodiment 22
[0649]The peptidomimetic macrocycle of any one of embodiments 1-20, wherein the peptidomimetic macrocycle comprises an amino acid sequence that has at least 60% identity to any one of SEQ ID NOs.: 707-757.
Embodiment 23
[0650]The peptidomimetic macrocycle of any one of embodiments 1-20, wherein the peptidomimetic macrocycle comprises an amino acid sequence that has at least 60% identity to any one of SEQ ID NOs.: 912-922.
Embodiment 24
[0651]The peptidomimetic macrocycle of any one of embodiments 1-20, wherein the peptidomimetic macrocycle comprises an amino acid sequence that has at least 60% identity to any one of SEQ ID NOs.: 1600-1625.
Embodiment 25
[0652]The peptidomimetic macrocycle of any one of embodiments 1-23, wherein the peptidomimetic macrocycle comprises an amino acid sequence that has at least 60% identity to any one of SEQ ID NOs.: 12, 755, and 920.
Embodiment 26
[0653]The peptidomimetic macrocycles of any one of embodiments 1-25, wherein WH is an amino acid with a side chain of the formula:
[0000]
[0000]wherein:
- X is alkylene, CH, CH2, NRα, O, or S, wherein Rαis —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl;
- Rais H, CN, or C(O)CH3;
- Rbis H, methyl, ethyl, allyl, propyl, isopropyl, butyl, or isobutyl;
- each Rc, Rd, and Reis independently —H, C1-C4saturated or unsaturated, straight or branched, hydrocarbon chain, or an electron-withdrawing group, wherein at least one of Rc, Rd, and Reis an electron withdrawing group;
- Rfis halogen, a C2alkynyl or alkenyl side chain optionally substituted with oxo, halogen, NO2, or CN; and
- n′ iso, 1, 2, 3, 4, or 5.
Embodiment 27
[0660]The peptidomimetic macrocycles of any one of embodiments 1-25, wherein WH is an amino acid with a side chain of the formula:
[0000]
[0661]wherein:
- X is alkylene, CH, CH2, NRα, O, or S, wherein Rαis —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl; and
- each Rc, Rd, and Reis independently —H, C1-C4saturated or unsaturated, straight or branched, hydrocarbon chain, or an electron-withdrawing group, wherein at least one of Rc, Rd, and Reis an electron withdrawing group.
Embodiment 28
[0664]The peptidomimetic macrocycle of any one of embodiments 1-25, wherein WH is an amino acid with a side chain of the formula:
[0000]
Embodiment 29
[0665]The peptidomimetic macrocycles of any one of embodiments 1-26, wherein WH is an amino acid with a side chain of the formula:
[0000]
Embodiment 30
[0666]The peptidomimetic macrocycles of any one of embodiments 1-26, wherein WH is an amino acid with a side chain of the formula:
[0000]
[0000]wherein:
- X is alkylene, CH, CH2, NRα, O, or S, wherein Rαis —H, alkyl, alkenyl, alkynyl, arylalkyl, cycloalkyl, heteroalkyl, cycloalkylalkyl, heterocycloalkyl, cycloaryl, or heterocycloaryl;
- each Rc, Rd, and Reis independently —H, C1-C4saturated or unsaturated, straight or branched, hydrocarbon chain, or an electron-withdrawing group, wherein at least one of Rc, Rd, and Reis an electron withdrawing group; and
- n′ is 0, 1, 2, 3, 4, or 5.
Embodiment 31
[0670]The peptidomimetic macrocycle of any one of embodiments 1-26, wherein WH is an amino acid with a side chain of the formula:
[0000]
[0000]wherein each Rc, Rd, and Reis independently —H, C1-C4saturated or unsaturated, straight or branched, hydrocarbon chain, or an electron-withdrawing group, wherein at least one of Rc, Rd, and Reis an electron withdrawing group; and n′ is 0, 1, 2, 3, 4, or 5.
Embodiment 32
[0671]A pharmaceutical composition comprising a peptidomimetic macrocycle of any one of embodiments 1-31 and a pharmaceutically-acceptable carrier.
Embodiment 33
[0672]A method of treating a disorder, the method comprising administering to a subject in need thereof a therapeutically-effective amount of the peptidomimetic macrocycle of any one of embodiments 1-31.
Embodiment 34
[0673]The method of embodiment 33, wherein the disorder is a cancer.
Embodiment 35
[0674]The method of embodiments 33 or 34, wherein the cancer is a solid cancer.
Embodiment 36
[0675]The method of embodiments 33 or 34, wherein the cancer is a liquid cancer.
Embodiment 37
[0676]The method of any one of embodiments 33-36, wherein the cancer is resistant to a BCL-2 inhibitor therapy.
Embodiment 38
[0677]The method of any one of embodiments 33-37, wherein the BCL-2 inhibitor therapy is navitoclax or obatoclax.
Embodiment 39
[0678]The method of any one of embodiments 33-35, 37, or 38, wherein the cancer is a lymphoma.
Embodiment 40
[0679]The method of any one of embodiments 33-35 or 37-39, wherein the cancer is B-cell lymphoma.
Embodiment 41
[0680]The method of any one of embodiments 33-40, wherein the administration is intravenous.
Embodiment 42
[0681]The method of any one of embodiments 33-40, wherein the administration is subcutaneous.
Embodiment 43
[0682]The method of any one of embodiments 33-40, wherein the administration is oral.
Embodiment 44
[0683]The method of any one of embodiments 33-43, further comprising administering to the subject a therapeutically-effective amount of a BCL-2 inhibitor.
Embodiment 45
[0684]The method of any one of embodiments 33-44, wherein the BCL-2 inhibitor is obatoclax.
Embodiment 46
[0685]The method of any one of embodiments 33-44, wherein the BCL-2 inhibitor is venetoclax.
Embodiment 47
[0686]The method of any one of embodiments 33-44, wherein the BCL-2 inhibitor is navitoclax.










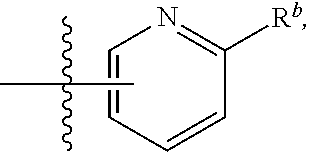
















 ” when used as part of a molecular structure refers to a single bond or a trans or cis double bond.
” when used as part of a molecular structure refers to a single bond or a trans or cis double bond.




































 ” is [D]v, [E]was defined above, and n is an integer between 0 and 20, 50, 100, 200, 300, 400 or 500. In some embodiments, the substituent “n” shown in the preceding paragraph is 0. In other embodiments, the substituent “n” shown in the preceding paragraph is less than 50, 40, 30, 20, 10, or 5.
” is [D]v, [E]was defined above, and n is an integer between 0 and 20, 50, 100, 200, 300, 400 or 500. In some embodiments, the substituent “n” shown in the preceding paragraph is 0. In other embodiments, the substituent “n” shown in the preceding paragraph is less than 50, 40, 30, 20, 10, or 5.