FIELD OF THE INVENTION
[0001]The present invention relates to an active substance combination comprising at least one compound with 5-HT6receptor affinity, and at least one N-methyl-D-aspartate-receptor ligand (NMDA-receptor ligand), a medicament comprising said active substance combination, and the use of said active substance combination for the manufacture of a medicament.
BACKGROUND
[0002]Cognitive and/or degenerative brain disorders are characterized clinically by progressive loss of memory, cognition, reasoning, judgement and emotional stability that gradually leads to profound mental deterioration and ultimately death. In an example of such disorders, Alzheimer's disease is a common cause of progressive mental failure (dementia) in aged humans and is believed to represent the fourth most common medical cause of death in the United States. In particular, Alzheimer's disease is associated with degeneration of cholinergic neurons in the basal forebrain that play a fundamental role in cognitive functions, including memory. Cognitive and/or degenerative brain disorders have been observed in varied races and ethnic groups world-wide and present a major public health problem. These diseases are currently estimated to affect about two to three million individuals in the United States alone and the occurrence will increase world-wide as the human life span increases.
[0003]Cognitive and/or degenerative brain disorders are incurable with presently used medications, however, the symptoms of these disorders seem to be possibly alleviated by using compounds such as memantine.
[0004]Whereas known compounds which act as NMDA-receptor ligands are generally effective for treating disorders related to NMDA-receptors such as cognitive disorders, in particular for treating Alzheimer's disease, in some instances they show undesirable side effects. Specifically, many of these compounds that have been tested in humans can cause potentially serious side effects such as gastrointestinal complications including insomnia, restlessness, headache, akathisia, fatigue, nausea, emesis, ulcers, constipation, flatulence, diarrhea, hypertension, respiratory depression and psychological and physical dependence.
[0005]Therefore, there is a need to provide a medicament suitable for the prophylaxis and/or treatment of disorders related to NMDA-receptors and to 5-HT6receptors, which preferably does not show the undesired side effects of the conventional compounds which act as NMDA-receptor ligands, or at least less frequent and/or less pronounced.
BRIEF DESCRIPTION OF THE INVENTION
[0006]The authors of the present invention have developed a medicament suitable for the prophylaxis and/or treatment of cognitive disorders, in particular for treating Alzheimer's disease, which does not show, or at least reduced significantly, the undesired side effects mentioned above of the conventional medicaments.
[0007]Therefore, a first aspect of the present invention relates to an active substance combination comprising:
[0000](A) at least one compound with 5-HT6receptor affinity,
and
(B) at least one NMDA-receptor ligand.
[0008]It has surprisingly been found that the compounds with 5-HT6receptor affinity and the compounds which act as NMDA-receptor ligands show a synergistic effect in their pharmacological activities. Consequently, the dose of the corresponding compounds may be reduced in comparison to the dose necessary for an individual administration of said compounds.
[0009]According to the invention it has also been found that the action of a NMDA-receptor antagonist potentiates the action of the compound with 5-HT6receptor affinity, so the combination of a NMDA-receptor antagonist and a compound with 5-HT6receptor affinity for use in the treatment of disorders that are related to NMDA-receptors, and to 5-HT6receptors may result in a faster onset of action and an increased success rate. The invention therefore particularly resides in the combined action of a NMDA-receptor compound, particularly an antagonist, and a compound with 5-HT6receptor affinity, or the dual action of a substance possessing both NMDA-receptor antagonist activity and 5-HT6receptor affinity, for the treatment of disorders that are related to NMDA-receptors, and/or to 5-HT6receptors.
[0010]In another aspect, the present invention relates to a medicament comprising the active substance combination as defined above and optionally one or more pharmacologically acceptable adjuvants.
[0011]A third aspect of the invention refers to a medicament as defined above, for simultaneous NMDA-receptor inhibition and 5-HT6-receptor regulation.
[0012]Also, another aspect of the invention relates to the use of the active substance combination in the manufacture of a medicament for simultaneous NMDA-receptor inhibition and 5-HT6-receptor regulation.
[0013]Another aspect of the invention refers to a pharmaceutical formulation which comprises the active substance combination and optionally one or more pharmacologically acceptable adjuvants.
[0014]Finally, the present invention also relates to a method for simultaneous NMDA-receptor inhibition and 5-HT6-receptor regulation, said method comprises administering to a patient in need of such a treatment a therapeutically effective amount of an active substance combination as defined above.
BRIEF DESCRIPTION OF THE DRAWINGS
[0015]FIG. 1 shows the results obtained for the novel object discrimination paradigm trial in rats, when they have been administered intraperitoneally with different doses of memantine (0, 5, 10, 15 and 20 mg/kg).
[0016]FIG. 2 shows the results obtained for the novel object discrimination paradigm trial in rats, when they have been administered intraperitoneally with the compound 841 alone, with memantine alone, or with a combination of compound 841 and memantine.
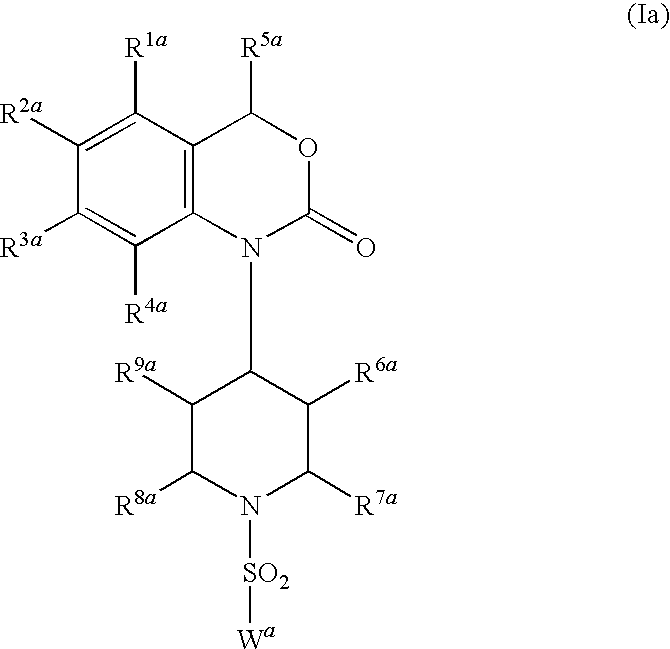
DETAILED DESCRIPTION OF THE INVENTION
[0017]In the treatment of cognitive disorders, the effect on e.g. memory or novel object discrimination is significantly greater in the group that is treated with a combination of at least one NMDA-receptor antagonist and at least one compound with 5-HT6receptor affinity than in the group that is treated with at least one NMDA-receptor antagonist or at least one compound with 5-HT6receptor affinity exclusively.
[0018]Also there is an indication that in the treatment of depression, the effect on the symptoms—in an animal model—is more pronounced in the group that is treated with a combination of at least one NMDA-receptor antagonist and at least one compound with 5-HT6receptor affinity than in the group that is treated with at least one NMDA-receptor antagonist or at least one compound with 5-HT6receptor affinity exclusively.
[0019]In one embodiment of the present invention the binding of compounds present as component (A) to the 5-HT6-receptor is determined by a K, value of less than 7000 nM, particularly preferably of less than 6500 nM, more particularly preferably of less than 200 nM, more particularly preferably of less than 100 nM.
[0020]In one embodiment of the present invention, the compounds present as component (B) act as NMDA-receptor antagonists. The NMDA-receptor antagonist of the invention may be any ligand that binds to and inhibits the NMDA-receptor, thereby resulting in a biological response. The potential of a given substance to act as a NMDA-receptor antagonist may be determined using standard in vitro binding assays and/or standard in vivo functionality tests.
[0021]In one embodiment of the present invention the binding of compounds present as component (B) to the NMDA-receptor is determined by an ECsoor IC50value of less than 300 μM, preferably less than 100 μM, when determined in a standard functionality assay using a mouse, rat, or human NMDA-receptor ion channel.
[0022]In one embodiment the NMDA-receptor antagonist blocks the NMDA receptor at the PCP binding site
[0023]In another embodiment of the present invention as component (A) at least one compound is present, which is selected from the group consisting of the benzoxazinone-derived sulfonamide compounds of general formula (Ia)
[0000]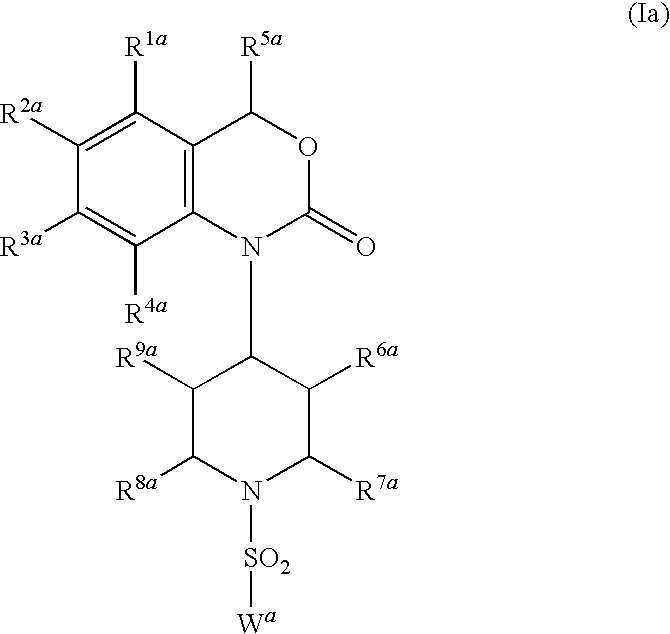
[0000]wherein
R1a, R2a, R3aand R4a, independently of one another, each represent a hydrogen atom; halogen; an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as ring member containing cycloaliphatic radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; an unsubstituted or at least mono-substituted aryl- or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; nitro; cyano; —O—R10a; —O—(C═O)—R11a; —(C═O)—OR11a; —SR12a; —SOR12a; —SO2R12a; —NH—SO2R12a; —SO2NH2or —NR13aR14a;
R5arepresents a hydrogen atom; an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical or a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as ring member containing cycloaliphatic radical;
R6a, R7a, R8a, R9a, independently of one another, each represent a hydrogen atom; an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as ring member containing cycloaliphatic radical; a cyano group or a —C(═O)—OR15amoiety;
Warepresents an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as ring member containing cycloaliphatic radical, which may be bonded via an optionally mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene or alkenylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
a —NR16aR17amoiety, or
a —C(═O)—R18amoiety;
R10arepresents a hydrogen atom; an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as ring member containing cycloaliphatic radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R11arepresents a hydrogen atom; an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as ring member containing cycloaliphatic radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R12arepresents an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as ring member containing cycloaliphatic radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R13aand R14a, independently of one another, each represent a hydrogen atom; an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as ring member containing cycloaliphatic radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
or R13aand R14atogether with the bridging nitrogen atom form a saturated, unsaturated or aromatic heterocyclic ring, which is unsubstituted or at least mono-substituted and/or which may contain at least one further heteroatom as a ring member;
R15arepresents a hydrogen atom; an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as ring member containing cycloaliphatic radical or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R16arepresents an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
R17arepresents an unbranched or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical, and
R18arepresents an unsubstituted or at least mono-substituted aryl radical;
optionally in form of one of its stereoisomers, preferably enantiomers or diastereomers, its racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a solvate, respectively.
[0024]Preferred compounds of general formula (Ia) are those, wherein
[0000]R1a, R2a, R3aand R4a, independently of one another, each represent a hydrogen atom; a fluorine atom; a chlorine atom; a bromine atom; a methyl group or a methoxy group;
R5arepresents a hydrogen atom;
R6a, R7a, R8aand R9aeach represent a hydrogen atom;
Warepresents
an alkyl radical selected from the group consisting of methyl; ethyl; n-propyl; isopropyl; n-butyl; sec-butyl; isobutyl and tert-butyl; vinyl (CH2═CH—); —N(CH3)2; 1-naphthyl; benzyl; 2-naphtyl; phenyl; 2-methyl-phenyl; 3-methyl-phenyl; 4-methyl-phenyl; 2-ethyl-phenyl; 3-ethyl-phenyl; 4-ethyl-phenyl; 2-n-propyl-phenyl; 3-n-propyl-phenyl; 4-n-propyl-phenyl; 2-isopropyl-phenyl; 3-isopropyl-phenyl; 4-isopropyl-phenyl; 2-n-butyl-phenyl; 3-n-butyl-phenyl; 4-n-butyl-phenyl; 2-isobutyl-phenyl; 3-isobutyl-phenyl; 4-isobutyl-phenyl; 2-tert-butyl-phenyl; 3-tert-butyl-phenyl; 4-tert-butyl-phenyl; 1,1-dimethylpropyl-phenyl; 2-cyclopentyl-phenyl; 3-cyclopentyl-phenyl; 4-cyclopentyl-phenyl 2-cyclohexyl-phenyl; 3-cyclohexyl-phenyl; 4-cyclohexyl-phenyl; 2-methoxy-phenyl; 3-methoxy-phenyl; 4-methoxy-phenyl; 2-ethoxy-phenyl; 3-ethoxy-phenyl; 4-ethoxy-phenyl; 2-n-propoxy-phenyl; 3-n-propoxy-phenyl; 4-n-propoxy-phenyl; 2-iso-propoxy-phenyl; 3-iso-propoxy-phenyl; 4-isopropoxy-phenyl; 2-fluoro-phenyl; 3-fluoro-phenyl; 4-fluoro-phenyl; 2-chloro-phenyl; 3-chloro-phenyl; 4-chloro-phenyl; 2-bromo-phenyl; 3-bromo-phenyl; 4-bromo-phenyl; 2-trifluoromethyl-phenyl; 3-trifluoromethyl-phenyl; 4-trifluoromethyl-phenyl; 2-trifluoromethoxy-phenyl; 3-trifluoromethoxy-phenyl; 4-trifluoromethoxy-phenyl; 2-carboxy-phenyl; 3-carboxy-phenyl; 4-carboxy-phenyl; 2-acetyl-phenyl; 3-acetyl-phenyl; 4-acetyl-phenyl; 2-(C═O)—O—CH3-phenyl; 3-(C═O)—O—CH3-phenyl; 4-(C═O)—O—CH3-phenyl; 2-(CH2)—(CH2)—(C═O)—O—CH3-phenyl; 3-(CH2)—(CH2)—(C═O)—O—CH3-phenyl; 4-(CH2)—(CH2)—(C═O)—O—CH3-phenyl; 2-cyano-phenyl; 3-cyano-phenyl; 4-cyano-phenyl; 2-nitro-phenyl; 3-nitro-phenyl; 4-nitro-phenyl; 4-(4-bromophenoxy)-phenyl; 2-methylsulfonyl-phenyl; 3-methylsulfonyl-phenyl; 4-methylsulfonyl-phenyl; 2-phenyl-phenyl (biphenyl-2-yl); 3-phenyl-phenyl (biphenyl-3-yl); 4-phenyl-phenyl (biphenyl-4-yl); 2-phenoxy-phenyl; 3-phenoxy-phenyl; 4-phenoxy-phenyl; 2,4-dimethyl-phenyl; 3,4-dimethyl-phenyl; 2,4,6-trimethyl-phenyl; 2,3,5,6-tetramethyl-phenyl; pentamethyl-phenyl; 2,5-dimethoxy-phenyl; 3,4-dimethoxy-phenyl; 2,3-dichloro-phenyl; 2,4-dichloro-phenyl; 2,5-dichloro-phenyl; 3,4-dichloro-phenyl; 3,5-dichloro-phenyl; 2,6-dichloro-phenyl; 2,4-difluoro-phenyl; 3,4-difluoro-phenyl; 2,5-difluoro-phenyl; 2,6-difluoro-phenyl; 3-chloro-2-fluoro-phenyl; 3-chloro-4-fluoro-phenyl; 5-chloro-2-fluoro-phenyl; 2,3,4-trichloro-phenyl; 2,4,5-trichloro-phenyl; 2,4,6-trichloro-phenyl; 2,4,5-trifluoro-phenyl; 2,3,4-trifluoro-phenyl-; 2-chloro-4,5-difluoro-phenyl; 2-bromo-4-fluoro-phenyl; 2-bromo-4,6-difluoro-phenyl; 4-chloro-2,5-difluoro-phenyl; 5-chloro-2,4-difluoro-phenyl; 4-bromo-2,5-difluoro-phenyl; 5-bromo-2,4-difluoro-phenyl; pentafluoro-phenyl; 2,4-dinitro-phenyl; 4-chloro-3-nitro-phenyl; 2-methyl-5-nitro-phenyl; 5-bromo-2-methoxy-phenyl; 3-chloro-2-methyl-phenyl; 4-bromo-3-methyl-phenyl; 4-chloro-2,5-dimethyl-phenyl; 4-fluoro-3-methyl-phenyl; 5-fluoro-2-methyl-phenyl; 2-nitro-4-trifluoromethyl-phenyl; 2-methoxy-4-methyl-phenyl; 3,5-dichloro-2-hydroxy-phenyl; 3,5-dichloro-4-hydroxy-phenyl; 5-chloro-2,4-difluoro-phenyl; 3-chloro-4-(NH)—(C═O)—CH3-phenyl; 2-chloro-6-methyl-phenyl; 2-chloro-5-trifluoromethyl-phenyl; 2-chloro-5-trifluoromethoxy-phenyl; 4-bromo-2-trifluoromethoxy-phenyl; 4-bromo-2-trifluoromethyl-phenyl; 4-bromo-3-trifluoromethyl-phenyl; 3-carboxy-4-fluoro-phenyl; 3-carboxy-4-chloro-6-fluoro-phenyl; 4-methoxy-2,3,6-trimethyl-phenyl-; or one of the following groups:
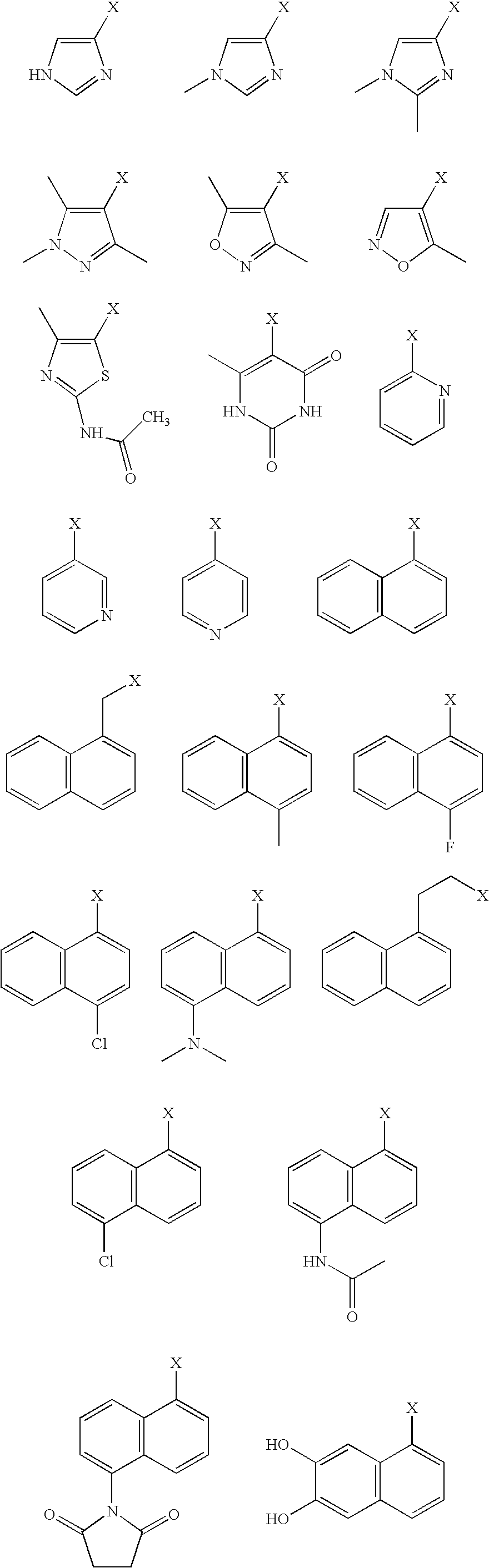
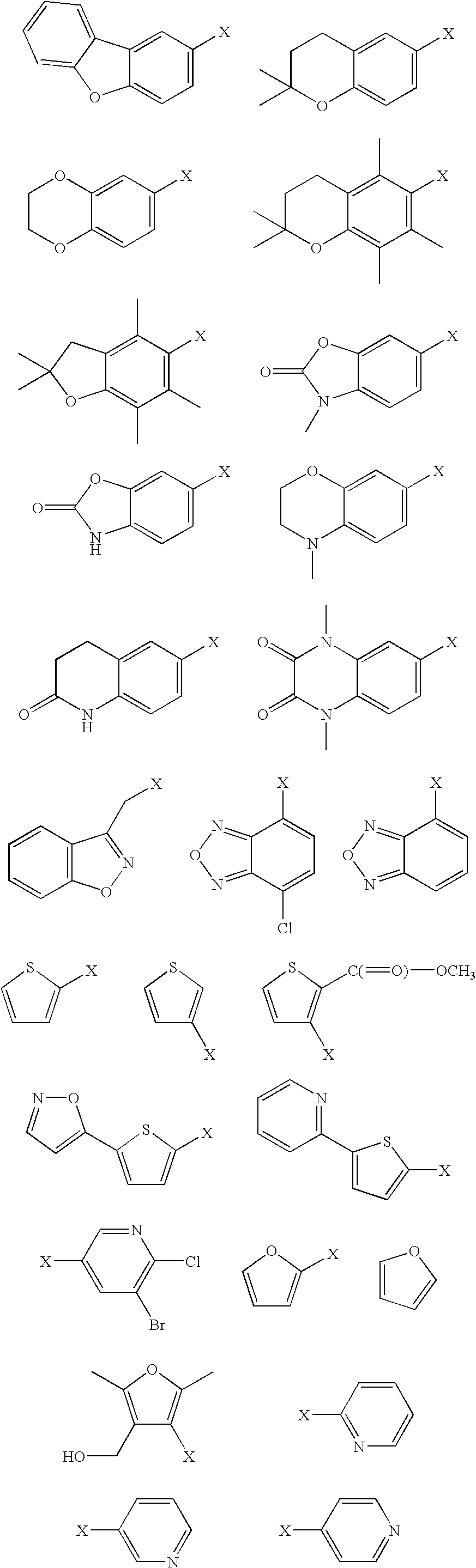
[0000]whereby in each case X denotes the position by which the respective substituent Wais bonded to the —SO2group of formula (Ia);
optionally in form of one of its stereoisomers, preferably enantiomers or diastereomers, its racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a solvate, respectively.
[0025]Preferred compounds of general formula (Ia) are those selected from the group consisting of:
- 1 1-[1-(Naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 2 1-[1-(Toluene-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 3 1-(1-Phenylmethanesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 4 1-(1-Benzenesulfonyl-piperidin-4-yl)-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 5 6-Chloro-1-[1-(toluene-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 6 6-Chloro-1-(1-phenylmethanesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 7 6-Chloro-1-[1-(naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 8 6-Chloro-1-[1-(naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 9 6-Chloro-1-[1-(5-chloro-3-methyl-benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 10 1-[1-(Thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 11 1-[1-(4-Acetyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 12 2-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzonitrile
- 13 1-[1-(2,4-Dimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 14 1-[1-(4-Methoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 15 1-[1-(2-Naphthalen-1-yl-ethanesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 16 8-Methyl-1-[1-(thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 17 1-[1-(4-Acetyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 18 2-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzonitrile
- 19 1-[1-(2,4-Dimethyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 20 1-[1-(4-Methoxy-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 21 8-Methyl-1-[1-(2-naphthalen-1-yl-ethanesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 22 4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonic acid dimethylamide
- 23 2-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid methyl ester
- 24 1-[1-(3-Trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 25 2-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid methyl ester
- 26 8-Methyl-1-[1-(3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 27 1-[1-(4-Acetyl-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 28 2-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzonitrile
- 29 6-Chloro-1-[1-(4-methoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 30 2-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid methyl ester
- 31 6-Chloro-1-[1-(2,4-dimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 32 6-Chloro-1-[1-(3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 33 1-[1-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 34 1-{1-[4-(4-Bromo-phenoxy)-benzenesulfonyl]-piperidin-4-yl}-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 35 1-[1-(4-Fluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 36 8-Methyl-1-[1-(naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 37 8-Methyl-1-(1-phenylmethanesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 38 1-[1-(4-Bromo-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 39 6-Chloro-1-[1-(4-methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 40 1-[1-(Butane-1-sulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 41 1-[1-(4-Bromo-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 42 1-[1-(4-Methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 43 1-[1-(Butane-1-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 44 6-Chloro-1-[1-(2-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 45 6-Chloro-1-[1-(3-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 46 1-[1-(Biphenyl-4-sulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 47 8-Methyl-1-[1-(2-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 48 8-Methyl-1-[1-(3-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 49 1-[1-(Biphenyl-4-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 50 8-Methyl-1-[1-(4-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 51 6-Chloro-1-[1-(4-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 52 1-(1-Ethanesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 53 1-[1-(Propane-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 54 1-[1-(Propane-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 55 6-Chloro-1-(1-ethanesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 56 6-Chloro-1-[1-(propane-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 57 6-Chloro-1-[1-(propane-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 58 6-Chloro-1-[1-(quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 59 1-[1-(4-Nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 60 6-Methyl-1-[1-(quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 61 6-Methyl-1-[1-(2-naphthalen-1-yl-ethanesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 62 6-Methyl-1-[1-(toluene-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 63 1-[1-(4-Fluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 64 6-Methyl-1-[1-(naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 65 6-Methyl-1-[1-(naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 66 1-[1-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 67 6-Methyl-1-[1-(4-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 68 1-(1-Benzenesulfonyl-piperidin-4-yl)-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 69 1-[1-(4-Chloro-3-nitro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 70 1-[1-(5-Dimethylamino-naphthalene-1-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 71 1-[1-(4-Chloro-3-nitro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 72 1-[1-(4-Chloro-3-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 73 6-Chloro-1-[1-(4-chloro-3-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 74 6-Chloro-1-[1-(5-dimethylamino-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 75 1-[1-(4-Methoxy-2,3,6-trimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 76 1-[1-(4-Methoxy-2,3,6-trimethyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 77 6-Chloro-1-[1-(4-methoxy-2,3,6-trimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 78 1-[1-(4-Methoxy-2,3,6-trimethyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 79 1-[1-(2-Bromo-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 80 1-[1-(2-Bromo-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 81 1-[1-(2-Bromo-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 82 1-[1-(2-Bromo-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 83 6-Chloro-1-[1-(2,3-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 84 1-[1-(2,3-Dichloro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 85 1-[1-(2,4,5-Trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 86 8-Methyl-1-[1-(2,4,5-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 87 6-Chloro-1-[1-(2,4,5-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 88 6-Methyl-1-[1-(2,4,5-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 89 1-[1-(5-Bromo-2-methoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 90 1-[1-(5-Bromo-2-methoxy-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 91 1-[1-(5-Bromo-2-methoxy-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 92 1-[1-(5-Bromo-2-methoxy-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 93 1-[1-(2,5-Dimethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 94 1-[1-(2,5-Dimethoxy-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 95 6-Chloro-1-[1-(2,5-dimethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 96 1-[1-(2,5-Dimethoxy-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 97 1-(1-Pentamethylbenzenesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 98 8-Methyl-1-(1-pentamethylbenzenesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 99 6-Chloro-1-(1-pentamethylbenzenesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 100 6-Methyl-1-(1-pentamethylbenzenesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 101 1-{1-[2-(2,2,2-Trifluoro-acetyl)-1,2,3,4-tetrahydro-isoquinoline-7-sulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 102 8-Methyl-1-{1-[2-(2,2,2-trifluoro-acetyl)-1,2,3,4-tetrahydro-isoquinoline-7-sulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 103 6-Chloro-1-{1-[2-(2,2,2-trifluoro-acetyl)-1,2,3,4-tetrahydro-isoquinoline-7-sulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 104 6-Methyl-1-{1-[2-(2,2,2-trifluoro-acetyl)-1,2,3,4-tetrahydro-isoquinoline-7-sulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 105 1-[1-(2-Methyl-5-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 106 8-Methyl-1-[1-(2-methyl-5-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 107 6-Chloro-1-[1-(2-methyl-5-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 108 6-Methyl-1-[1-(2-methyl-5-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 109 1-[1-(4-Bromo-2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 110 1-[1-(4-Bromo-2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 111 1-[1-(4-Bromo-2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 112 1-[1-(4-Bromo-2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 113 1-[1-(4-Chloro-2,5-dimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 114 1-[1-(4-Chloro-2,5-dimethyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 115 6-Chloro-1-[1-(4-chloro-2,5-dimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 116 1-[1-(4-Chloro-2,5-dimethyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 117 1-[1-(4-Methoxy-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 118 1-[1-(4-Isopropyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 119 1-[1-(4-Isopropyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 120 6-Chloro-1-[1-(4-isopropyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 121 1-[1-(4-Isopropyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 122 1-[1-(3-Chloro-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 123 1-[1-(3-Chloro-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 124 6-Chloro-1-[1-(3-chloro-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 125 1-[1-(3-Chloro-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 126 1-[1-(4-Bromo-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 127 6-Methyl-1-[1-(3-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 128 6-Methyl-1-[1-(3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 129 1-[1-(4-Trifluoromethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 130 1-[1-(2-Nitro-4-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 131 1-[1-(3-Fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 132 1-[1-(2,4-Dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 133 1-[1-(2,4,6-Trimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 134 1-[1-(2-Trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 135 8-Methyl-1-[1-(4-trifluoromethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 136 8-Methyl-1-[1-(2-nitro-4-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 137 1-[1-(3-Fluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 138 1-[1-(2,4-Dichloro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 139 8-Methyl-1-[1-(2,4,6-trimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 140 8-Methyl-1-[1-(2-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 141 1-[1-(4-Fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 142 1-[1-(4-Bromo-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 143 1-[1-(3-Nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 144 1-{1-[4-(4-Bromo-phenoxy)-benzenesulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 145 1-[1-(3-Methoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 146 1-[1-(2-Nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 147 8-Methyl-1-[1-(toluene-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 148 1-(1-Benzenesulfonyl-piperidin-4-yl)-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 149 1-[1-(3-Methoxy-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 150 1-[1-(2,4-Dimethyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 151 1-{1-[4-(4-Bromo-phenoxy)-benzenesulfonyl]-piperidin-4-yl}-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 152 6-Methyl-1-[1-(thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 153 1-[1-(Toluene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 154 1-[1-(5-Fluoro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 155 1-[1-(4-Isopropoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 156 1-[1-(3-Chloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 157 1-[1-(3,4-Dimethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 158 1-(1-Pentafluorobenzenesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 159 8-Methyl-1-[1-(toluene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 160 1-[1-(5-Fluoro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydrobenzo[d][1,3]oxazin-2-one
- 161 1-[1-(4-Isopropoxy-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 162 1-[1-(3-Chloro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 163 1-[1-(3,4-Dimethoxy-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 164 8-Methyl-1-(1-pentafluorobenzenesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 165 6-Methyl-1-[1-(toluene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 166 1-[1-(5-Fluoro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 167 1-[1-(4-Isopropoxy-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 168 1-[1-(3-Chloro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 169 1-[1-(3,4-Dimethoxy-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 170 6-Methyl-1-(1-pentafluorobenzenesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 171 6-Methyl-1-[1-(4-trifluoromethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 172 6-Methyl-1-[1-(2-nitro-4-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 173 1-[1-(3-Fluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 174 1-[1-(2,4-Dichloro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 175 6-Methyl-1-[1-(2,4,6-trimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 176 6-Methyl-1-[1-(2-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 177 1-[1-(3-Methoxy-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 178 6-Methyl-1-[1-(2-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 179 1-[1-(4-Acetyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 180 1-[1-(4-Methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 181 6-Methyl-1-(1-phenylmethanesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 182 2-[4-(6-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]benzoic acid methyl ester
- 183 6-Methyl-1-[1-(2-oxo-2H-chromene-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 184 6-Chloro-1-[1-(4-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 185 6-Chloro-1-[1-(3,5-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 186 1-{1-[4-(4-Bromo-phenoxy)-benzenesulfonyl]-piperidin-4-yl}-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 187 6-Chloro-1-[1-(thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 188 6-Chloro-1-[1-(3-methoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 189 6-Chloro-1-[1-(2-oxo-2H-chromene-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 190 6-Chloro-1-[1-(toluene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 191 6-Chloro-1-[1-(5-fluoro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 192 6-Chloro-1-[1-(4-isopropoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 193 6-Chloro-1-[1-(3-chloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 194 6-Chloro-1-[1-(3,4-dimethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 195 6-Chloro-1-(1-pentafluorobenzenesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 196 6-Chloro-1-[1-(4-trifluoromethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 197 6-Chloro-1-[1-(2-nitro-4-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 198 6-Chloro-1-[1-(3-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 199 6-Chloro-1-[1-(2,4-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 200 6-Chloro-1-[1-(2,4,6-trimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 201 6-Chloro-1-[1-(2-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 202 1-[1-(2-Oxo-2H-chromene-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 203 1-[1-(3,5-Dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 204 1-[1-(2,5-Dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 205 1-[1-(5-Bromo-6-chloro-pyridine-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 206 1-[1-(4-Chloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 207 1-[1-(2,6-Dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 208 8-Methyl-1-[1-(2-oxo-2H-chromene-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 209 1-[1-(3,5-Dichloro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 210 1-[1-(2,5-Dichloro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 211 1-[1-(5-Bromo-6-chloro-pyridine-3-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 212 1-[1-(4-Chloro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 213 1-[1-(2,6-Dichloro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 214 1-[1-(Biphenyl-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 215 6-Chloro-1-[1-(2,5-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 216 1-[1-(5-Bromo-6-chloro-pyridine-3-sulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 217 6-Chloro-1-[1-(4-chloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 218 6-Chloro-1-[1-(2,6-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 219 1-[1-(Biphenyl-4-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 220 2-[4-(6-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzonitrile
- 221 1-[1-(2,5-Dichloro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 222 1-[1-(5-Bromo-6-chloro-pyridine-3-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 223 1-[1-(4-Chloro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 224 1-[1-(2,6-Dichloro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 225 1-[1-(3,5-Dichloro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 226 6-Methyl-1-[1-(1-methyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 227 1-[1-(5-Bromo-2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 228 1-[1-(4-Methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 229 1-[1-(1-Methyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 230 1-[1-(5-Bromo-2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 231 1-[1-(6-Chloro-imidazo[2,1-b]thiazole-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 232 1-[1-(4-Ethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 233 1-[1-(Benzo[b]thiophene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 234 1-[1-(6-Chloro-imidazo[2,1-b]thiazole-5-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 235 1-[1-(4-Ethyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 236 1-[1-(Benzo[b]thiophene-3-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 237 6-Chloro-1-[1-(6-chloro-imidazo[2,1-b]thiazole-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 238 6-Chloro-1-[1-(4-ethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 239 1-[1-(Benzo[b]thiophene-3-sulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 240 1-[1-(6-Chloro-imidazo[2,1-b]thiazole-5-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 241 1-[1-(4-Ethyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 242 1-[1-(Benzo[b]thiophene-3-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 243 1-[1-(7-Chloro-benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 244 1-[1-(2-Methoxy-4-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 245 3-{4-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-phenyl}-propionic acid methyl ester
- 246 1-[1-(2,4-Dinitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 247 1-[1-(7-Chloro-benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 248 1-[1-(2-Methoxy-4-methyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 249 3-{4-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-phenyl}-propionic acid methyl ester
- 250 1-[1-(2,4-Dinitro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 251 1-[1-(7-Chloro-benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 252 1-[1-(2-Methoxy-4-methyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 253 3-{4-[4-(6-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-phenyl}-propionic acid methyl ester
- 254 1-[1-(2,4-Dinitro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 255 6-Chloro-1-[1-(7-chloro-benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 256 6-Chloro-1-[1-(2-methoxy-4-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 257 3-{4-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-phenyl}-propionic acid methyl ester
- 258 6-Chloro-1-[1-(2,4-dinitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 259 6-Chloro-1-[1-(1-methyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 260 1-[1-(5-Bromo-2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 261 8-Methyl-1-[1-(1-methyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 262 1-[1-(5-Bromo-2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 263 1-[1-(Benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 264 1-[1-(Benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 265 1-[1-(Benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 266 1-[1-(Benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 267 1-[1-(2,5-Difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 268 1-[1-(2,5-Difluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 269 6-Chloro-1-[1-(2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 270 1-[1-(2,5-Difluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 271 1-[1-(4-Chloro-2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 272 1-[1-(4-Chloro-2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 273 6-Chloro-1-[1-(4-chloro-2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 274 1-[1-(4-Chloro-2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 275 1-[1-(2,4,5-Trifluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 276 8-Methyl-1-[1-(2,4,5-trifluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 277 6-Chloro-1-[1-(2,4,5-trifluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 278 6-Methyl-1-[1-(2,4,5-trifluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 279 1-[1-(3,5-Dichloro-2-hydroxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 280 1-[1-(2,6-Difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 281 1-[1-(2,6-Difluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 282 6-Chloro-1-[1-(2,6-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 283 1-[1-(2,6-Difluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 284 1-[1-(5-Chloro-2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 285 1-[1-(5-Chloro-2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 286 6-Chloro-1-[1-(5-chloro-2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 287 1-[1-(5-Chloro-2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 288 1-[1-(2-Chloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 289 1-[1-(2-Chloro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 290 6-Chloro-1-[1-(2-chloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 291 1-[1-(2-Chloro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 292 6-Chloro-1-[1-(2-naphthalen-1-yl-ethanesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 293 6-Bromo-1-[1-(4-bromo-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 294 6-Bromo-1-[1-(toluene-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 295 6-Bromo-1-[1-(2,4-dimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 296 6-Bromo-1-[1-(2-naphthalen-1-yl-ethanesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 297 6-Bromo-1-[1-(quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 298 6-Bromo-1-[1-(5-chloro-3-methyl-benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 299 6-Bromo-1-[1-(3-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 300 6-Bromo-1-[1-(naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 301 6-Bromo-1-[1-(naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 302 1-(1-Benzenesulfonyl-piperidin-4-yl)-6-bromo-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 303 6-Bromo-1-{1-[4-(4-bromo-phenoxy)-benzenesulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 304 6-Bromo-1-[1-(thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 305 6-Bromo-1-[1-(2-methyl-5-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 306 6-Bromo-1-[1-(4-bromo-2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 307 6-Bromo-1-[1-(toluene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 308 6-Bromo-1-[1-(5-fluoro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 309 6-Bromo-1-[1-(4-isopropoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 310 6-Bromo-1-[1-(3-chloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 311 6-Bromo-1-[1-(3,4-dimethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 312 6-Bromo-1-(1-pentafluorobenzenesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 313 6-Bromo-1-[1-(4-chloro-2,5-dimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 314 6-Bromo-1-[1-(3-methoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 315 6-Bromo-1-[1-(4-isopropyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 316 6-Bromo-1-[1-(4-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 317 6-Bromo-1-[1-(3-chloro-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 318 6-Bromo-1-(1-pentamethylbenzenesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 319 6-Bromo-1-[1-(2-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 320 6-Bromo-1-[1-(4-chloro-3-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 321 6-Bromo-1-[1-(5-dimethylamino-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 322 6-Bromo-1-[1-(4-nitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 323 1-[1-(4-Acetyl-benzenesulfonyl)-piperidin-4-yl]-6-bromo-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 324 6-Bromo-1-[1-(4-methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 325 1-[1-(Biphenyl-4-sulfonyl)-piperidin-4-yl]-6-bromo-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 326 6-Bromo-1-(1-phenylmethanesulfonyl-piperidin-4-yl)-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 327 6-Bromo-1-[1-(2,5-dimethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 328 6-Bromo-1-{1-[2-(2,2,2-trifluoro-acetyl)-1,2,3,4-tetrahydro-isoquinoline-7-sulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 329 6-Bromo-1-[1-(2,3-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 330 6-Bromo-1-[1-(2,4,5-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 331 6-Bromo-1-[1-(5-bromo-2-methoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 332 6-Bromo-1-[1-(4-trifluoromethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 333 6-Bromo-1-[1-(2-nitro-4-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 334 6-Bromo-1-[1-(3-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 335 6-Bromo-1-[1-(2,4-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 336 6-Bromo-1-[1-(2,4,6-trimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 337 6-Bromo-1-[1-(2-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 338 6-Bromo-1-[1-(2-bromo-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 339 6-Bromo-1-[1-(4-methoxy-2,3,6-trimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 340 1-[1-(3,5-Dichloro-4-hydroxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 341 1-[1-(3,5-Dichloro-4-hydroxy-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 342 6-Chloro-1-[1-(3,5-dichloro-4-hydroxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 343 1-[1-(3,5-Dichloro-4-hydroxy-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 344 6-Bromo-1-[1-(3,5-dichloro-4-hydroxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 345 6-Chloro-1-[1-(3,5-dichloro-2-hydroxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 346 6-Bromo-1-[1-(3,5-dichloro-2-hydroxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 347 2-[4-(6-Bromo-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzonitrile
- 348 6-Bromo-1-[1-(4-methoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 349 2-[4-(6-Bromo-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid methyl ester
- 350 6-Bromo-1-[1-(3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 351 6-Bromo-1-[1-(2-oxo-2H-chromene-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 352 6-Bromo-1-[1-(3,5-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 353 6-Bromo-1-[1-(2,5-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 354 6-Bromo-1-[1-(5-bromo-6-chloro-pyridine-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 355 6-Bromo-1-[1-(4-chloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 356 6-Bromo-1-[1-(2,6-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 357 6-Bromo-1-[1-(1-methyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 358 6-Bromo-1-[1-(5-bromo-2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 359 6-Bromo-1-[1-(4-ethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 360 6-Bromo-1-[1-(6-chloro-imidazo[2,1-b]thiazole-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 361 1-[1-(Benzo[b]thiophene-3-sulfonyl)-piperidin-4-yl]-6-bromo-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 362 6-Bromo-1-[1-(7-chloro-benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 363 6-Bromo-1-[1-(2-methoxy-4-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 364 3-{4-[4-(6-Bromo-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-phenyl}-propionic acid methyl ester
- 365 6-Bromo-1-[1-(2,4-dinitro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 366 1-[1-(Benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-6-bromo-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 367 6-Bromo-1-[1-(2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 368 6-Bromo-1-[1-(4-chloro-2,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 369 6-Bromo-1-[1-(2,4,5-trifluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 370 6-Bromo-1-[1-(2,6-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 371 6-Bromo-1-[1-(5-chloro-2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 372 6-Bromo-1-[1-(2-chloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 373 6-Bromo-1-[1-(2,3,4-trifluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 374 N-{4-[4-(6-Bromo-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-2-chloro-phenyl}-acetamide
- 375 1-[1-(2,3,4-Trifluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 376 8-Methyl-1-[1-(2,3,4-trifluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 377 6-Chloro-1-[1-(2,3,4-trifluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 378 6-Methyl-1-[1-(2,3,4-trifluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 379 N-{2-Chloro-4-[4-(6-methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-phenyl}-acetamide
- 380 1-[1-(3,4-Difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 381 1-[1-(3,4-Difluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 382 6-Chloro-1-[1-(3,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 383 1-[1-(3,4-Difluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 384 6-Bromo-1-[1-(3,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 385 N-{2-Chloro-4-[4-(8-methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-phenyl}-acetamide
- 386 1-[1-(2-Chloro-4,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 387 1-[1-(2-Chloro-4,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 388 6-Chloro-1-[1-(2-chloro-4,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 389 1-[1-(2-Chloro-4,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 390 6-Bromo-1-[1-(2-chloro-4,5-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 391 N-{2-Chloro-4-[4-(2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-phenyl}-acetamide
- 392 1-[1-(Benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 393 1-[1-(Benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 394 1-[1-(Benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 395 1-[1-(Benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 396 1-[1-(Benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-6-bromo-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 397 N-{2-Chloro-4-[4-(6-chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-phenyl}-acetamide
- 398 1-[1-(Benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 399 1-[1-(Benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 400 1-[1-(Benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 401 1-[1-(Benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 402 1-[1-(Benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-6-bromo-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 403 1-(1-Ethanesulfonyl-piperidin-4-yl)-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 404 1-[1-(2,4-Difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 405 1-[1-(2,4-Difluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 406 6-Chloro-1-[1-(2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 407 1-[1-(2,4-Difluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 408 6-Bromo-1-[1-(2,4-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 409 8-Methyl-1-[1-(propane-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 410 1-[1-(3,4-Dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 411 1-[1-(3,4-Dichloro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 412 6-Chloro-1-[1-(3,4-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 413 1-[1-(3,4-Dichloro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 414 6-Bromo-1-[1-(3,4-dichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 415 8-Methyl-1-[1-(propane-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 416 1-[1-(2-Chloro-6-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 417 1-[1-(2-Chloro-6-methyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 418 6-Chloro-1-[1-(2-chloro-6-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 419 1-[1-(2-Chloro-6-methyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 420 1-[1-(2-Chloro-6-methyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 421 8-Methyl-1-[1-(2,3,5,6-tetramethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 422 1-[1-(2,3,4-Trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 423 8-Methyl-1-[1-(2,3,4-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 424 6-Chloro-1-[1-(2,3,4-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 425 6-Methyl-1-[1-(2,3,4-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 426 6-Bromo-1-[1-(2,3,4-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 427 1-[1-(2,3,5,6-Tetramethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 428 1-[1-(Thiophene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 429 8-Methyl-1-[1-(thiophene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 430 6-Chloro-1-[1-(thiophene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 431 6-Methyl-1-[1-(thiophene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 432 6-Bromo-1-[1-(thiophene-3-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 433 6-Chloro-1-[1-(2,3,5,6-tetramethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 434 1-[1-(2,4,6-Trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 435 8-Methyl-1-[1-(2,4,6-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 436 6-Chloro-1-[1-(2,4,6-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 437 6-Methyl-1-[1-(2,4,6-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 438 6-Bromo-1-[1-(2,4,6-trichloro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 439 6-Methyl-1-[1-(2,3,5,6-tetramethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 440 1-[1-(2-Bromo-4,6-difluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 441 1-[1-(2-Bromo-4,6-difluoro-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 442 1-[1-(2-Bromo-4,6-difluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 443 6-Bromo-1-[1-(2-bromo-4,6-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 444 6-Bromo-1-[1-(2,3,5,6-tetramethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 445 1-[1-(4-Bromo-2-trifluoromethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 446 1-[1-(4-Bromo-2-trifluoromethoxy-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 447 1-[1-(4-Bromo-2-trifluoromethoxy-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 448 1-[1-(4-Bromo-2-trifluoromethoxy-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 449 6-Bromo-1-[1-(4-bromo-2-trifluoromethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 450 1-[1-(4-Phenoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 451 1-[1-(3-Bromo-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 452 1-[1-(3-Bromo-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 453 1-[1-(3-Bromo-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 454 1-[1-(3-Bromo-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 455 6-Bromo-1-[1-(3-bromo-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 456 8-Methyl-1-[1-(4-phenoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 457 1-[1-(4-tert-Butyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 458 1-[1-(4-tert-Butyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 459 1-[1-(4-tert-Butyl-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 460 1-[1-(4-tert-Butyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 461 6-Bromo-1-[1-(4-tert-butyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 462 6-Chloro-1-[1-(4-phenoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 463 1-[1-(2-Bromo-4,6-difluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 464 1-[1-(2-Methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 465 6-Chloro-1-[1-(2-methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 466 1-[1-(2-Methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 467 6-Bromo-1-[1-(2-methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 468 8-Methyl-1-[1-(4-propyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 469 6-Chloro-1-[1-(4-propyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 470 6-Methyl-1-[1-(4-propyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 471 6-Bromo-1-[1-(4-propyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 472 1-[1-(3-Chloro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 473 6-Chloro-1-[1-(3-chloro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 474 1-[1-(3-Chloro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 475 6-Bromo-1-[1-(3-chloro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 476 1-[1-(4-Butyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 477 1-[1-(4-Butyl-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 478 1-[1-(4-Butyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 479 6-Bromo-1-[1-(4-butyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 480 1-[1-(4-Bromo-3-methyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 481 1-[1-(4-Bromo-3-methyl-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 482 1-[1-(4-Bromo-3-methyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 483 6-Bromo-1-[1-(4-bromo-3-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 484 1-{1-[4-(1,1-Dimethyl-propyl)-benzenesulfonyl]-piperidin-4-yl}-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 485 6-Chloro-1-{1-[4-(1,1-dimethyl-propyl)-benzenesulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 486 1-{1-[4-(1,1-Dimethyl-propyl)-benzenesulfonyl]-piperidin-4-yl}-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 487 6-Bromo-1-{1-[4-(1,1-dimethyl-propyl)-benzenesulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 488 1-(1-Ethenesulfonyl-piperidin-4-yl)-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 489 3-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 490 3-[4-(6-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 491 3-[4-(6-Bromo-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 492 1-[1-(3-Chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 493 6-Chloro-1-[1-(3-chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 494 1-[1-(3-Chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 495 6-Bromo-1-[1-(3-chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 496 N-{4-Methyl-5-[4-(8-methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-thiazol-2-yl}-acetamide
- 497 N-{5-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-4-methyl-thiazol-2-yl}-acetamide
- 498 N-{4-Methyl-5-[4-(6-methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-thiazol-2-yl}-acetamide
- 499 N-{5-[4-(6-Bromo-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-4-methyl-thiazol-2-yl}-acetamide
- 500 1-[1-(2-Bromo-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 501 1-[1-(2-Bromo-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 502 1-[1-(2-Bromo-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 503 6-Bromo-1-[1-(2-bromo-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 504 1-[1-(5-Chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 505 6-Chloro-1-[1-(5-chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 506 1-[1-(5-Chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 507 6-Bromo-1-[1-(5-chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 508 1-[1-(4-Bromo-3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 509 1-[1-(4-Bromo-3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 510 1-[1-(4-Bromo-3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 511 6-Bromo-1-[1-(4-bromo-3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 512 1-[1-(2-Methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 513 1-[1-(4-Propyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 514 1-[1-(3-Chloro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 515 1-[1-(4-Butyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 516 1-[1-(4-Bromo-3-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 517 1-{1-[4-(1,1-Dimethyl-propyl)-benzenesulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 518 N-{4-Methyl-5-[4-(2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-thiazol-2-yl}-acetamide
- 519 1-[1-(3-Chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 520 1-[1-(2-Bromo-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 521 1-[1-(4-Bromo-3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 522 1-[1-(5-Chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 523 1-[1-(Isoquinoline-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 524 6-Fluoro-1-[1-(2-methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 525 6-Fluoro-1-[1-(4-propyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 526 1-[1-(3-Chloro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 527 1-[1-(4-Butyl-benzenesulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 528 1-[1-(4-Bromo-3-methyl-benzenesulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 529 1-{1-[4-(1,1-Dimethyl-propyl)-benzenesulfonyl]-piperidin-4-yl}-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 530 N-{5-[4-(6-Fluoro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-4-methyl-thiazol-2-yl}-acetamide
- 531 1-[1-(3-Chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 532 1-[1-(2-Bromo-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 533 1-[1-(4-Bromo-3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 534 1-[1-(5-Chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 535 6-Fluoro-1-[1-(isoquinoline-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 536 6-Fluoro-1-[1-(quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 537 1-[1-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 538 6-Fluoro-1-[1-(naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 539 6-Fluoro-1-[1-(naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 540 1-[1-(Benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 541 1-[1-(Benzo[b]thiophene-3-sulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 542 8-Methoxy-1-[1-(quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 543 1-[1-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 544 8-Methoxy-1-[1-(naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 545 8-Methoxy-1-[1-(naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 546 1-[1-(Benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 547 1-[1-(Benzo[b]thiophene-3-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 548 5-Chloro-1-[1-(2-methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 549 5-Chloro-1-[1-(4-propyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 550 5-Chloro-1-[1-(3-chloro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 551 1-[1-(4-Butyl-benzenesulfonyl)-piperidin-4-yl]-5-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 552 1-[1-(4-Bromo-3-methyl-benzenesulfonyl)-piperidin-4-yl]-5-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 553 5-Chloro-1-{1-[4-(1,1-dimethyl-propyl)-benzenesulfonyl]-piperidin-4-yl}-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 554 N-{5-[4-(5-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-4-methyl-thiazol-2-yl}-acetamide
- 555 5-Chloro-1-[1-(3-chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 556 1-[1-(2-Bromo-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-5-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 557 1-[1-(4-Bromo-3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-5-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 558 5-Chloro-1-[1-(5-chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 559 5-Chloro-1-[1-(isoquinoline-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 560 1-[1-(2-Methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 561 1-[1-(2-Methanesulfonyl-benzenesulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 562 1-[1-(3-Chloro-2-methyl-benzenesulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 563 1-[1-(4-Butyl-benzenesulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 564 1-[1-(4-Bromo-3-methyl-benzenesulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 565 1-{1-[4-(1,1-Dimethyl-propyl)-benzenesulfonyl]-piperidin-4-yl}-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 566 N-{5-[4-(8-Methoxy-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-4-methyl-thiazol-2-yl}-acetamide
- 567 1-[1-(3-Chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 568 1-[1-(2-Bromo-4-fluoro-benzenesulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 569 1-[1-(4-Bromo-3-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 570 1-[1-(5-Chloro-2-fluoro-benzenesulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 571 1-[1-(Isoquinoline-5-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one; hydrochloride
- 572 1-[1-(4-Methyl-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 573 6-Chloro-1-[1-(4-methyl-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 574 6-Methyl-1-[1-(4-methyl-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 575 8-Methyl-1-[1-(4-methyl-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 576 6-Fluoro-1-[1-(4-methyl-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 577 8-Methoxy-1-[1-(4-methyl-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 578 5-Chloro-1-[1-(4-methyl-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 579 5-Chloro-1-[1-(naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 580 5-Chloro-1-[1-(naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 581 5-Chloro-1-[1-(quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 582 5-Chloro-1-[1-(5-chloro-3-methyl-benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 583 1-[1-(Benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-5-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 584 1-[1-(Benzo[b]thiophene-3-sulfonyl)-piperidin-4-yl]-5-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 585 6-Bromo-1-[1-(4-methyl-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 586 2-Chloro-4-fluoro-5-[4-(8-methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 587 2-Chloro-5-[4-(6-chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-4-fluoro-benzoic acid
- 588 2-Chloro-4-fluoro-5-[4-(6-methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 589 2-Chloro-4-fluoro-5-[4-(2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 590 2-Chloro-4-fluoro-5-[4-(8-methoxy-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 591 2-Chloro-5-[4-(5-chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-4-fluoro-benzoic acid
- 592 3-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 593 3-[4-(8-Methoxy-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 594 3-[4-(5-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 595 1-[1-(Isoquinoline-5-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one; hydrochloride
- 596 6-Chloro-1-[1-(isoquinoline-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one; hydrochloride
- 597-[1-(Isoquinoline-5-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one; hydrochloride
- 598 6,7-Difluoro-1-[1-(quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 599 1-[1-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 600 6,7-Difluoro-1-[1-(naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 601 6,7-Difluoro-1-[1-(naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 602 1-[1-(Benzo[b]thiophene-2-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 603 1-[1-(Benzo[b]thiophene-3-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 604 1-[1-(5-Dimethylamino-naphthalene-1-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 605 1-[1-(Biphenyl-4-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 606 1-[1-(Benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 607 1-[1-(Benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 608 1-[1-(7-Chloro-benzo[1,2,5]oxadiazole-4-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 609 6,7-Difluoro-1-[1-(4-methyl-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 610 1-[1-(4-Chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 611 1-[1-(4-Fluoro-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 612 1-[1-(Dibenzofuran-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 613 1-[1-(2,3-Dihydro-benzofuran-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 614 1-[1-(Biphenyl-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 615 1-[1-(5-Isoxazol-5-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 616 1-[1-(4-Chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 617 1-[1-(4-Fluoro-naphthalene-1-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 618 1-[1-(Dibenzofuran-2-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 619 1-[1-(2,3-Dihydro-benzofuran-5-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 620 1-[1-(Biphenyl-2-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 621 1-[1-(5-Isoxazol-5-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 622 5-Chloro-1-[1-(4-chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 623 5-Chloro-1-[1-(4-fluoro-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 624 5-Chloro-1-[1-(dibenzofuran-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 625 5-Chloro-1-[1-(2,3-dihydro-benzofuran-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 626 1-[1-(Biphenyl-2-sulfonyl)-piperidin-4-yl]-5-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 627 5-Chloro-1-[1-(5-isoxazol-5-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 628 1-[1-(4-Chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 629 1-[1-(4-Fluoro-naphthalene-1-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 630 1-[1-(Dibenzofuran-2-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 631 1-[1-(2,3-Dihydro-benzofuran-5-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 632 1-[1-(Biphenyl-2-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 633 1-[1-(5-Isoxazol-5-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 634 6-Chloro-1-[1-(4-chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 635 6-Chloro-1-[1-(4-fluoro-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 636 6-Chloro-1-[1-(dibenzofuran-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 637 6-Chloro-1-[1-(2,3-dihydro-benzofuran-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 638 1-[1-(Biphenyl-2-sulfonyl)-piperidin-4-yl]-6-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 639 6-Chloro-1-[1-(5-isoxazol-5-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 640 1-[1-(4-Chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 641 1-[1-(4-Fluoro-naphthalene-1-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 642 1-[1-(Dibenzofuran-2-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 643 1-[1-(2,3-Dihydro-benzofuran-5-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 644 1-[1-(Biphenyl-2-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 645 1-[1-(5-Isoxazol-5-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 646 1-[1-(4-Chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 647 6,7-Difluoro-1-[1-(4-fluoro-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 648 1-[1-(Dibenzofuran-2-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 649 1-[1-(2,3-Dihydro-benzofuran-5-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 650 1-[1-(Biphenyl-2-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 651 6,7-Difluoro-1-[1-(5-isoxazol-5-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 652 1-[1-(1,2-Dimethyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 653 1-[1-(5-Methyl-benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 654 1-[1-(3,5-Dimethyl-isoxazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 655 1-[1-(1,2-Dimethyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 656 8-Methyl-1-[1-(5-methyl-benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 657 1-[1-(3,5-Dimethyl-isoxazole-4-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 658 6-Chloro-1-[1-(1,2-dimethyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 659 6-Chloro-1-[1-(5-methyl-benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 660 6-Chloro-1-[1-(3,5-dimethyl-isoxazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 661 1-[1-(1,2-Dimethyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 662 8-Methoxy-1-[1-(5-methyl-benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 663 1-[1-(3,5-Dimethyl-isoxazole-4-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 664 5-Chloro-1-[1-(1,2-dimethyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 665 5-Chloro-1-[1-(5-methyl-benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 666 5-Chloro-1-[1-(3,5-dimethyl-isoxazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 667 1-[1-(1,2-Dimethyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 668 6-Methyl-1-[1-(5-methyl-benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 669 1-[1-(3,5-Dimethyl-isoxazole-4-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 670 1-[1-(1,2-Dimethyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 671 6-Fluoro-1-[1-(5-methyl-benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 672 1-[1-(3,5-Dimethyl-isoxazole-4-sulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 673 1-[1-(1,2-Dimethyl-1H-imidazole-4-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 674 6,7-Difluoro-1-[1-(5-methyl-benzo[1,2,5]thiadiazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 675 1-[1-(3,5-Dimethyl-isoxazole-4-sulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 676 1-[1-(5-Chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 677 1-[1-(5-Chloro-naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 678 N-{5-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-naphthalen-1-yl}-acetamide
- 679 1-[1-(5-Chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 680 1-[1-(5-Chloro-naphthalene-2-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 681 N-{5-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-naphthalen-1-yl}-acetamide
- 682 5-Chloro-1-[1-(5-chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 683 5-Chloro-1-[1-(5-chloro-naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 684 N-{5-[4-(5-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-naphthalen-1-yl}-acetamide
- 685 1-[1-(5-Chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 686 1-[1-(5-Chloro-naphthalene-2-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 687 N-{5-[4-(8-Methoxy-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-naphthalen-1-yl}-acetamide
- 688 2,5-Dimethyl-4-[4-(8-methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-furan-3-carboxylic acid methyl ester
- 689 8-Methyl-1-[1-(2-oxo-2,3-dihydro-benzothiazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 690 1-[1-(4-Fluoro-3-methyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 691 8-Methyl-1-[1-(2-oxo-2,3-dihydro-benzooxazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 692 1-[1-(4-Cyclohexyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 693 2,5-Dimethyl-4-[4-(2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-furan-3-carboxylic acid methyl ester
- 694 1-[1-(4-Fluoro-3-methyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 695 1-[1-(2-Oxo-2,3-dihydro-benzooxazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 696 1-[1-(4-Cyclohexyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 697 2-Fluoro-5-[4-(8-methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 698 2-Fluoro-5-[4-(2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzoic acid
- 699 1-[1-(2-Oxo-2,3-dihydro-benzothiazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 700 1-[1-(5-Pyridin-2-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 701 3-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzonitrile
- 702 3-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-thiophene-2-carboxylic acid methyl ester
- 703 1-{5-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-naphthalen-1-yl}-pyrrolidine-2,5-dione
- 704 1-[1-(2-Chloro-5-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 705 1-[1-(3,4-Dimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 706 8-Methyl-1-[1-(5-pyridin-2-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 707 3-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzonitrile
- 708 3-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-thiophene-2-carboxylic acid methyl ester
- 709 1-{5-[4-(8-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-naphthalen-1-yl}-pyrrolidine-2,5-dione
- 710 1-[1-(2-Chloro-5-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 711 1-[1-(3,4-Dimethyl-benzenesulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 712 5-Chloro-1-[1-(5-pyridin-2-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 713 3-[4-(5-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzonitrile
- 714 3-[4-(5-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-thiophene-2-carboxylic acid methyl ester
- 715 1-{5-[4-(5-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-naphthalen-1-yl}-pyrrolidine-2,5-dione
- 716 5-Chloro-1-[1-(2-chloro-5-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 717 5-Chloro-1-[1-(3,4-dimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 718 6-Methyl-1-[1-(5-pyridin-2-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 719 3-[4-(6-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzonitrile
- 720 3-[4-(6-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-thiophene-2-carboxylic acid methyl ester
- 721 1-{5-[4-(6-Methyl-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-naphthalen-1-yl}-pyrrolidine-2,5-dione
- 722 1-[1-(2-Chloro-5-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 723 1-[1-(3,4-Dimethyl-benzenesulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 724 6-Chloro-1-[1-(5-pyridin-2-yl-thiophene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 725 3-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-benzonitrile
- 726 3-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-thiophene-2-carboxylic acid methyl ester
- 727 1-{5-[4-(6-Chloro-2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-naphthalen-1-yl}-pyrrolidine-2,5-dione
- 728 6-Chloro-1-[1-(2-chloro-5-trifluoromethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 729 6-Chloro-1-[1-(3,4-dimethyl-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 730 1-[1-(5-Methyl-isoxazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 731 1-[1-(2,2-Dimethyl-chroman-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 732 1-[1-(4-Methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 733 1-[1-(2,3-Dihydro-benzo[1,4]dioxine-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 734 1-[1-(1,3,5-Trimethyl-1H-pyrazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 735 1-[1-(3-Methyl-2-oxo-2,3-dihydro-benzooxazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 736 8-Methyl-1-[1-(5-methyl-isoxazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 737 1-[1-(2,2-Dimethyl-chroman-6-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 738 8-Methyl-1-[1-(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 739 1-[1-(2,3-Dihydro-benzo[1,4]dioxine-6-sulfonyl)-piperidin-4-yl]-8-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 740 8-Methyl-1-[1-(1,3,5-trimethyl-1H-pyrazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 741 8-Methyl-1-[1-(3-methyl-2-oxo-2,3-dihydro-benzooxazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 742 8-Methoxy-1-[1-(1,3,5-trimethyl-1H-pyrazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 743 8-Methoxy-1-[1-(3-methyl-2-oxo-2,3-dihydro-benzooxazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 744 1-[1-(Benzo[d]isoxazol-3-ylmethanesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 745 1-[1-(2,2,4,6,7-Pentamethyl-2,3-dihydro-benzofuran-5-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 746 6-Methyl-5-[4-(2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-1H-pyrimidine-2,4-dione
- 747 1-[1-(3-Methyl-quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 748 1-[1-(2,2,5,7,8-Pentamethyl-chroman-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 749 1,4-Dimethyl-6-[4-(2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-1,4-dihydro-quinoxaline-2,3-dione
- 750 1-[1-(1H-Imidazole-4-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 751 1-[1-(2-Oxo-1,2,3,4-tetrahydro-quinoline-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 752 7-[4-(2-Oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-1,5-dihydro-benzo[b][1,4]diazepine-2,4-dione
- 753 8-Methyl-1-[1-(3-methyl-quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 754 6-Chloro-1-[1-(3-methyl-quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 755 5-Chloro-1-[1-(3-methyl-quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 756 8-Methoxy-1-[1-(3-methyl-quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 757 1-[1-(Pyridine-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 758 1-[1-(6,7-Dihydroxy-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 759 Acetic acid 3-acetoxy-5-[4-(2-oxo-4H-benzo[d][1,3]oxazin-1-yl)-piperidine-1-sulfonyl]-naphthalen-2-yl ester
- 760 1-[1-(1H-Benzoimidazole-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 761 1-[1-(1H-Benzoimidazole-2-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 762 1-[1-(1H-Benzoimidazole-2-sulfonyl)-piperidin-4-yl]-5-chloro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 763 1-[1-(2,5-Dimethoxy-benzenesulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 764 1-[1-(2,5-Dimethoxy-benzenesulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 765 1-[1-(2,5-Dimethoxy-benzenesulfonyl)-piperidin-4-yl]-6,7-difluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 766 5-Chloro-1-[1-(2,5-dimethoxy-benzenesulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 767 1-[1-(5-Dimethylamino-naphthalene-1-sulfonyl)-piperidin-4-yl]-8-methoxy-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 768 5-Chloro-1-[1-(5-dimethylamino-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 769 6-Chloro-1-[1-(5-chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 770 1-[1-(5-Chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 771 1-[1-(5-Chloro-naphthalene-1-sulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 772 6-Chloro-1-[1-(5-chloro-naphthalene-2-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 773 1-[1-(5-Chloro-naphthalene-2-sulfonyl)-piperidin-4-yl]-6-methyl-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 774 1-[1-(5-Chloro-naphthalene-2-sulfonyl)-piperidin-4-yl]-6-fluoro-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 775 6-Methyl-1-[1-(3-methyl-quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 776 6-Fluoro-1-[1-(3-methyl-quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 777 6,7-Difluoro-1-[1-(3-methyl-quinoline-8-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 778 6-Chloro-1-[1-(3-methyl-2-oxo-2,3-dihydro-benzooxazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 779 6-Methyl-1-[1-(3-methyl-2-oxo-2,3-dihydro-benzooxazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 780 6-Fluoro-1-[1-(3-methyl-2-oxo-2,3-dihydro-benzooxazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 781 6,7-Difluoro-1-[1-(3-methyl-2-oxo-2,3-dihydro-benzooxazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 782 5-Chloro-1-[1-(3-methyl-2-oxo-2,3-dihydro-benzo oxazole-6-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 783 6-Chloro-1-[1-(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 784 6-Methyl-1-[1-(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 785 6-Fluoro-1-[1-(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 786 8-Methoxy-1-[1-(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-one
- 787 5-Chloro-1-[1-(4-methyl-3,4-dihydro-2H-benzo[1,4]oxazine-7-sulfonyl)-piperidin-4-yl]-1,4-dihydro-benzo[d][1,3]oxazin-2-on;
optionally in form of one of its stereoisomers, preferably enantiomers or diastereomers, its racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a solvate, respectively.
[0813]The compounds of general formula (Ia) may be prepared according to the disclosure of WO 2005/014045.
[0814]In another embodiment, as component (A) at least one compound is present, which is selected from the group consisting of indole-derived sulfonamide compounds of general formula (Ib)
[0000]
[0000]wherein
R1brepresents a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical,
which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; a —(CH2)mb—NR13bR14bmoiety with mb=0, 1, 2, 3, 4 or 5; a —C(═O)—R8bmoiety; a —S(═O)2—R9bmoiety; or a —S(═O)2—C(H)AbBbmoiety;
R2brepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —NH2; —SH; —OH; —CN; —C(═O)—OH; —O—R10b; —S—R11b; —C(═O)—OR12b; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a chain member containing aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R3brepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —CN; —O—R10b; —S—R11b; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; a —CH(OC2H5)—CH2—NR13bR14bmoiety or a —(CH2)nb—NR13bR14bmoiety with nb=0, 1, 2, 3, 4 or 5; a —S(═O)2—R9bmoiety; a —S(═O)2—C(H)AbBbmoiety; or a —C(═O)—(CH2)pb—C(═O)—N-DbEbmoiety with pb=0, 1, 2, 3, 4 or 5;
R4b, R5b, R6band R7b, independently of one another, each represent a hydrogen atom; —NO2; —NH2; —SH; —OH; —CN; —C(═O)—OH; —C(═O)—H; —S(═O)2—OH; —C(═O)—NH2; —S(═O)2—NH2; —C(═O)—R8b; —S(═O)2—R9b; —O—R10b; —S—R11b; —C(═O)—OR12b; —N(R15b)—S(═O)2—R16b; —NH—R17b; —NR18bR19b; —C(═O)—NHR20b, —C(═O)—NR21bR22b; —S(═O)2—NHR23b; —S(═O)2—NR24bR25b; —O—C(═O)—R26b; —NH—C(═O)—R27b; —NR28b—C(═O)—R29b; NH—C(═O)—O—R30b; NR31b—C(═O)—O—R32b; —S(═O)2—O—R33b; a halogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
with the proviso that at least one of the substituents R4b, R5b, R6band R7brepresents a —N(R15b)—S(═O)2—R16bmoiety;
R8b, R12b, R17b, R18b, R19b, R20b, R21b, R22b, R23b, R24b, R25b, R26b, R27b, R28b, R29b, R30b, R31b, R32band R33b, independently of one another, each represent a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group;
R9brepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R10band R11b, independently of one another, each represent a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R13band R14b, independently of one another, each represent a hydrogen atom; or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or
R13band R14btogether with the bridging nitrogen form an unsubstituted or at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R15brepresents a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical or a —S(═O)2—R16bmoiety;
R16brepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
Aband Bbtogether with the bridging carbon form an unsubstituted or at least mono-substituted, saturated or unsaturated cycloaliphatic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
Dband Ebtogether with the bridging nitrogen form an unsubstituted or at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
or
Dband Eb, independently of one another, each represent a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[0815]In another embodiment of the present invention compounds of general formula (Ib) are selected from the group consisting of compounds of general formula (Ih)
[0000]
[0000]wherein
R1hrepresents a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or a —(CH2)mh—NR13hR14hmoiety with mh=0, 1, 2, 3, 4 or 5;
R2hrepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —CN; —O—R10h; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R3hrepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —CN; —O—R10h; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or a —(CH2)nh—NR13hR14hmoiety with nh=0, 1, 2, 3, 4 or 5;
R4h, R5hand R7h, independently of one another, each represent a hydrogen atom; —NO2; —CN; —O—R10h; —C(═O)—OR12h; a halogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R10hrepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R13hand R14h, independently of one another, each represent a hydrogen atom; or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or
R13hand R4htogether with the bridging nitrogen form an unsubstituted or at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R15hrepresents a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical or a —S(═O)2—R16hmoiety;
and R16hrepresents an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[0816]Particularly preferred compounds of general formula (Ih) are those, wherein
[0000]R1hrepresents a hydrogen atom or a —(CH2)mh—NR13hR14hradical,
R2h, and R7heach represent hydrogen,
R3hrepresents a hydrogen atom, 1-methyl-piperidin-4-yl or a —(CH2)nh—NR13hR14hmoiety with nh=0, 1 or 2,
R4hrepresents chlorine, bromine or a hydrogen atom,
R4hrepresents —C(═O)—O—C2H5or a hydrogen atom,
R15hrepresents hydrogen or a —S(═O)2—R16hmoiety,
R13hand R14h, identical or different, each represent methyl, ethyl, isopropyl or n-propyl, more preferably methyl,
or
R13hand R14h, together with the bridging nitrogen atom form a 5- or 6-membered heterocyclic ring, more preferably form a pyrrolidine ring or a piperidine ring
and
R16hrepresents an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, thiophenyl, benzo[b]furanyl, benzo[b]thiophenyl and imidazo[2,1-b]thiazolyl which may be substituted by 1, 2 or 3 substituents selected from the group consisting of chlorine, methyl, phenyl and —O-phenyl and/or which may be bonded via a C1-2alkylene group,
and mh is 0, 1 or 2,
optionally in form of one of its stereoisomers, preferably enantiomers or diastereomers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a salt thereof, preferably a corresponding, physiologically acceptable salt thereof, or a corresponding solvate thereof.
[0817]More particularly preferred compounds of general formula (Ih) are those selected from the group consisting of:
- [788] N-[1-(2-Dimethylamino ethyl)-1H-indol-6-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulfonamide,
- [789] N-[1-(2-Dimethylaminoethyl)-1H-indol-6-yl]-naphthalene-2-sulfonamide,
- [790] N-[1-(2-Dimethylaminoethyl)-1H-indol-6-yl]-naphthalene-1-sulfonamide,
- [791] N-[1-(2-Dimethylaminoethyl)-1H-indol-6-yl]-6-chloroimidazo[2,1-b]thiazole-5-sulfonamide,
- [792] N-[1-(2-Dimethylaminoethyl)-1H-indol-6-yl]-4-phenylbenzenesulfonamide,
- [793] N-[1-(2-Dimethylaminoethyl)-1H-indol-6-yl]-2-(naphthalene-1-yl)-ethanesulfonamide,
- [794] N-[1-(2-Dimethylaminoethyl)-1H-indol-6-yl]-4-phenoxybenzenesulfonamide,
- [795] N-[1-(2-Dimethylaminoethyl)-1H-indol-6-yl]-3,5-dichlorobenzenesulfonamide,
- [796] 5-Chloro-3-methyl-N-[1-[2-(pyrrolidin-1-yl)ethyl-1H-indol-6-yl]-benzo[b]thiophene-2-sulfonamide,
- [797] N-(1-[2-(Pyrrolidin-1-yl)ethyl]-1H-indol-6-yl]-napthyl-2-sulfonamide,
- [798] N-[1-[2-Pyrrolidin-1-yl]ethyl]-1H-indol-6-yl]-naphthalene-1-sulfonamide,
- [799] 6-Chloro-N-[1-[2-(pyrrolidin-1-yl)ethyl]-1H-indol-6-yl]-imidazo[2,1-b]thiazole-5-sulfonamide,
- [800] 4-Phenyl-N-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indol-6-yl)-benzenesulfonamide
- [801] 2-(Naphth-1-yl)-N-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indol-6-yl)-ethansulfonamide,
- [802] 4-Phenoxy-N-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indol-6-yl)-benzenesulfonamide
- [803] 3,5-Dichloro-N-(1-(2-(pyrrolidin-1-yl)-1H-indol-6-yl)-benzenesulfonamide,
- [804] 5-chloro-N-(3-(2-(diethylamino)ethyl)-1H-indol-6-yl)-3-methylbenzo[b]thiophene-2-sulfonamide,
- [805] N-(3-(2-(diethylamino)ethyl)-1H-indol-6-yl)naphthalene-2-sulfonamide,
- [806] N-(3-(2-(diethylamino)ethyl)-1H-indol-6-yl)naphthalene-1-sulfonamide,
- [807] 6-chloro-N-(3-(2-(diethylamino)ethyl)-1H-indol-6-yl)imidazo[2,1-b]thiazole-5-sulfonamide,
- [808] N-(3-(2-(diethylamino)ethyl)-1H-indol-6-yl)-4-phenylbenzenesulfonamide,
- [809] N-(3-(2-(diethylamino)ethyl)-1H-indol-6-yl)-4-phenoxybenzenesulfonamide,
- [810] 3,5-dichloro-N-(3-(2-(diethylamino)ethyl)-1H-indol-6-yl)benzenesulfonamide,
- [811] 4,5-dichloro-N-(3-(2-(diethylamino)ethyl)-1H-indol-6-yl)thiophene-2-sulfonamide,
- [812] 5-chloro-N-(3-(2-(diethylamino)ethyl)-1H-indol-6-yl)naphthalene-1-sulfonamide,
- [813] 5-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-6-yl)-3-methylbenzo[b]thiophene-2-sulfonamide,
- [814] N-(3-(2-(dimethylamino)ethyl)-1H-indol-6-yl)naphthalene-2-sulfonamide,
- [815] N-(3-(2-(dimethylamino)ethyl)-1H-indol-6-yl)naphthalene-1-sulfonamide,
- [816] 6-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-6-yl)imidazo[2,1-b]thiazole-5-sulfonamide,
- [817] N-(3-(2-(dimethylamino)ethyl)-1H-indol-6-yl)-4-phenylbenzenesulfonamide,
- [818] N-(3-(2-(dimethylamino)ethyl)-1H-indol-6-yl)-2-(naphthalen-1-yl)ethanesulfonamide,
- [819] N-(3-(2-(dimethylamino)ethyl)-1H-indol-6-yl)-4-phenoxybenzenesulfonamide,
- [820] 3,5-dichloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-6-yl)benzenesulfonamide,
- [821] 4,5-dichloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-6-yl)thiophene-2-sulfonamide,
- [822] 5-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-6-yl)naphthalene-1-sulfonamide,
- [823] 6-bis(6-chloroimidazo[2,1-b]thiazol-5-ylsulfonyl)amino-3-(2-(dimethylamino)ethyl)-1H-indole,
- [824] 6-bis(3,5-dichlorobenzenesulfonyl)amino-3-(2-(dimethylamino)ethyl)-1H-indole,
- [825] 6-b is (4,5-dichlorothiophene-2-sulfonyl)amino-3-(2-(dimethylamino)ethyl)-1H-indole,
- [826] 6-bis(5-chloro-3-methylbenzo[b]thiophene-2-sulfonyl)amino-3-(2-(dimethylamino)-1-ethoxyethyl)-1H-indole
- [827] Ethyl 6-(5-chloro-3-methylbenzo[b]thiophene-2-sulfonamido)-3-(1-methylpiperidin-4-yl)-1H-indole-5-carboxylate,
- [828] N-(4-bromo-3-(1-methylpiperidin-4-yl)-1H-indol-6-yl)naphthalene-1-sulfonamide,
- [829] N-(7-bromo-3-(2-(dimethylamino)ethyl)-1H-indol-5-yl)benzofuran-2-sulfonamide,
- [830] N-(7-methoxy-3-(2-(pyrrolidin-1-yl)ethyl)-1H-indol-5-yl)benzo[c][1,2,5]thiadiazole-4-sulfonamide,
- [831] N-(7-methoxy-3-(2-(pyrrolidin-1-yl)ethyl)-1H-indol-5-yl)naphthalene-2-sulfonamide and
- [832] 6-chloro-N-(7-methoxy-3-(2-(pyrrolidin-1-yl)ethyl)-1H-indol-5-yl)imidazo[2,1-b]thiazole-5-sulfonamide;
and their corresponding salts and solvates.
[0863]The compounds of general formula (Ih) may be prepared according to the disclosure of WO 2005/013976 and WO 2006/024535.
[0864]In another embodiment of the present invention compounds of general formula (Ib) are selected from the group consisting of compounds of general formula (Ik)
[0000]
[0000]wherein
R1krepresents a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; an unsubstituted or at least mono-substituted phenyl radical or an unsubstituted or at least mono-substituted benzyl radical;
R3krepresents a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or a —(CH2)nk—NR13kR14kmoiety with nk=0, 1, 2, 3, 4 or 5;
R13kand R14k, independently of one another, each represent a hydrogen atom; or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or
R13kand R14ktogether with the bridging nitrogen form an unsubstituted or at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R15krepresents a hydrogen atom; or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
and R16krepresents an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[0865]Preferred compounds of general formula (Ik) are those, wherein
[0000]nk represents 0, 1, 2, 3 or 4;
R1krepresents hydrogen,
R3krepresents a —NR13kR14kmoiety or a moiety selected from the group consisting of
[0000]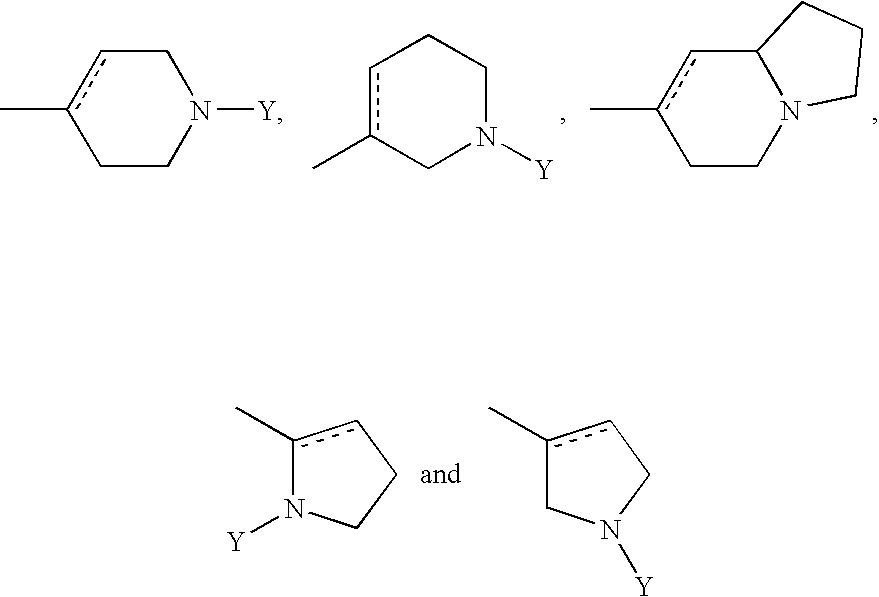
[0000]wherein, if present, the dotted line represents an optional chemical bond and Y represents hydrogen, a methyl group or an ethyl group,
R15krepresents hydrogen, a methyl group or an ethyl group,
R13kand R14k, identical or different, represent a methyl group, an ethyl group, an n-propyl group, an isopropyl group, an n-butyl group, an isobutyl group, a sec-butyl group, or a tert-butyl group, or
R13kand R14ktogether with the bridging nitrogen atom form a moiety selected from the group consisting of
[0000]
[0000]wherein Z represents hydrogen, a methyl group or an ethyl group,
R16krepresents a moiety selected from the group consisting of
[0000]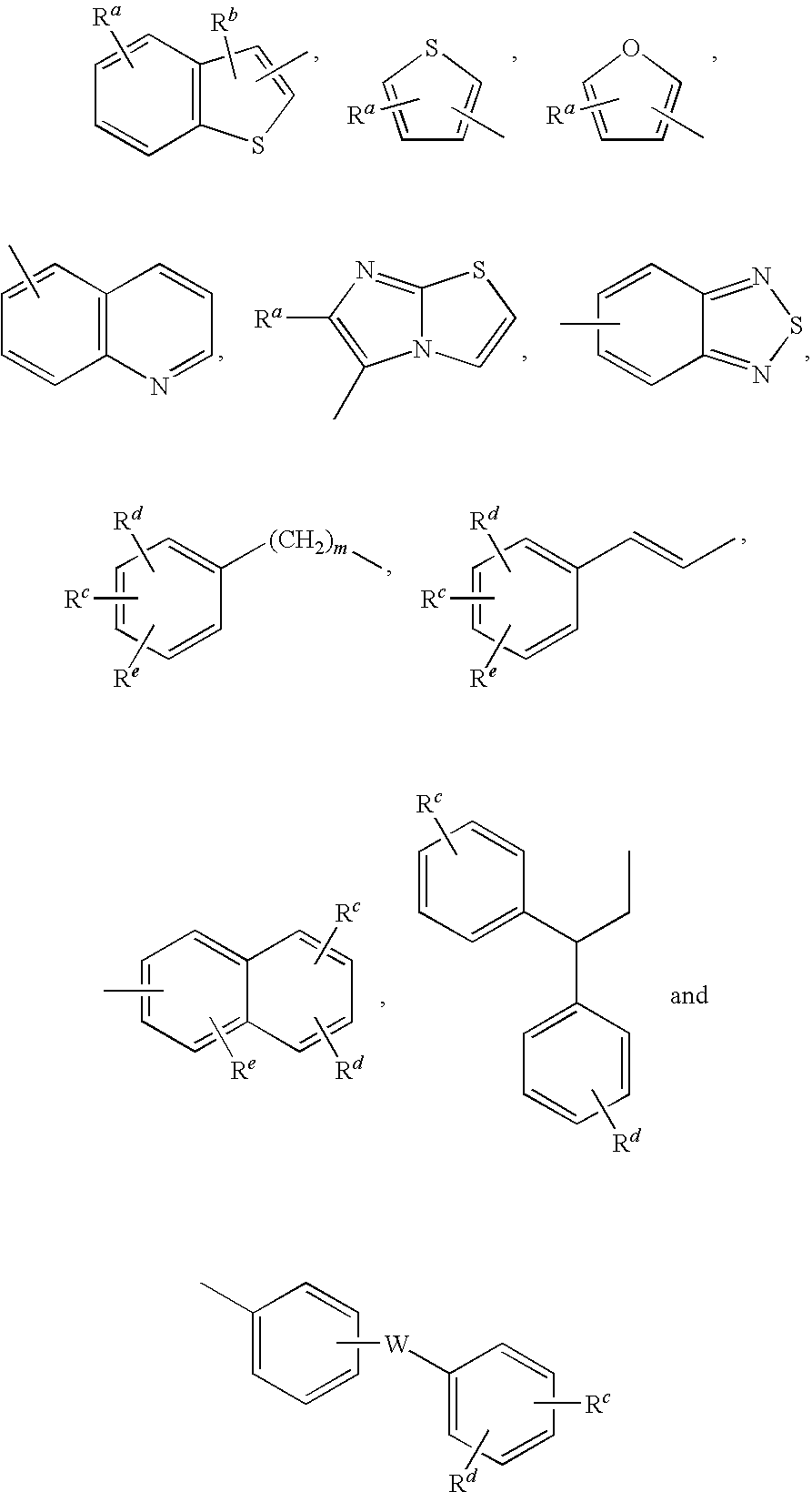
[0000]wherein
Raand Rbare each independently selected from the group consisting of hydrogen, fluorine, chlorine, bromine, methyl, ethyl, pyridinyl, thiophenyl and furyl,
Rc, Rdand Reare each independently selected from the group consisting of hydrogen, fluorine, chlorine, bromine, methyl, ethyl, methoxy, ethoxy and —CF3,
W represents a single chemical bond between the two rings, a CH2-group, O, S or a NRf-moiety, wherein Rfis hydrogen, methyl or ethyl,
m is 0, 1, 2, 3 or 4;
optionally in form of one of its stereoisomers, preferably enantiomers or diastereomers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a salt thereof, preferably a corresponding, physiologically acceptable salt thereof, or a corresponding solvate thereof.
[0866]Particularly preferred compounds of general formula (Ik) are those selected from the group consisting of:
- [833] N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [834] N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]naphthalene-1-sulphonamide,
- [835] Hydrochloride N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]naphthalene-1-sulphonamide,
- [836] N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]-3,5-dichlorobenzenesulphonamide,
- [837] N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]-4-phenylbenzenesulphonamide,
- [838] N-[3-(2-diethylamino ethyl)-1H-indol-5-yl]-5-chlorothiophene-2-sulphonamide,
- [839] N-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [840] N-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]naphthalene-1-sulphonamide,
- [841] N-[3-(2-dimethylamino-ethyl)-1H-indol-5-yl]-6-chloroimidazo[2,1-b]thiazol-5-sulphonamide,
- [842] N-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [843] N-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide hydrochloride,
- [844] N-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]naphthalene-1-sulphonamide,
- [845] N-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]naphthalene-1-sulphonamide hydrochloride,
- [846] N-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]-5-chlorothiophene-2-sulphonamide,
- [847] N-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]-4-phenylbenzenesulphonamide,
- [848] N-[3-(1-methylpiperidin-4-yl)-1H-indol-5-yl]quinoline-8-sulphonamide,
- [849] N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]naphthalene-2-sulphonamide,
- [850] N-[3-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-1H-indol-5-yl]naphthalene-1-sulphonamide,
- [851] N-[3-(4-methylpiperazin-1-yl)methyl-1H-indol-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [852] N-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-5-(2-pyridil)thiophene-2-sulphonamide,
- [853] N-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-2,1,3-benzothiadiazol-4-sulphonamide,
- [854] N-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]quinoline-8-sulphonamide,
- [855] N-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-5-chloronaphthalene-2-sulphonamide,
- [856] N-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-4-phenoxybenzenesulphonamide,
- [857] N-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-4-phenylbenzenesulphonamide,
- [858] N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]-N-ethyl-naphthalene-2-sulphonamide,
- [859] N-{3-[2-(morpholin-4-yl)ethyl]-1H-indol-5-yl}-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [860] N-{3-[2-(morpholin-4-yl)ethyl]-1H-indol-5-yl}naphthalene-1-sulphonamide,
- [861] N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]naphthalene-2-sulphonamide,
- [862] N-[3-dimethylaminomethyl-1H-indol-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [863] N-[3-(2-dipropylaminoethyl)-1H-indol-5-yl]naphthalene-1-sulphonamide,
- [864] N-[3-(2-dipropylaminoethyl)-1H-indol-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [865] N-[3-(2-dibutylaminoethyl)-1H-indol-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [866] N-[3-(2-dibutylaminoethyl)-1H-indol-5-yl]naphthalene-1-sulphonamide,
- [867] N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]-5-chloronaphthalene-1-sulphonamide,
- [868] N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]-trans-β-styrenesulphonamide,
- [869] N-[3-(4-methylpiperazin-1-yl)methyl-1H-indol-5-yl]-trans-β-styrenesulphonamide,
- [870] N-[3-(octahydroindolizin-7-yl)-1H-indol-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [871] N-[3-(2-diethylaminoethyl)-1H-indol-5-yl]-6-chloroimidazo[2,1-b]thiazol-5-sulphonamide,
- [872] N-{3-[2-(morpholin-4-yl)ethyl]-1H-indol-5-yl}naphthalene-2-sulphonamide,
- [873] N-[3-(4-methylpiperazin-1-yl)methyl-1H-indol-5-yl]-α-toluenesulphonamide,
- [874] N-[3-(3-diethylaminopropyl)-1H-indol-5-yl]naphthalene-2-sulphonamide,
- [875] N-[3-(3-diethylaminopropyl)-1H-indol-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [876] N-{3-[2-(pyrrolidin-1-yl)ethyl]-1H-indol-5-yl}-5-chloro-3-methylbenzo[b]thiophene-2-sulphonamide,
- [877] N-{3-[2-(pyrrolidin-1-yl)ethyl]-1H-indol-5-yl}naphthalene-1-sulphonamide,
- [878] N-{3-[2-(pyrrolidin-1-yl)ethyl]-1H-indol-5-yl}naphthalene-2-sulphonamide,
- [879] N-[3-(2-dipropylaminoethyl)-1H-indol-5-yl]naphthalene-2-sulphonamide,
- [880] N-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-5-chloronaphthalene-1-sulphonamide,
- [881] N-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]naphthalene-2-sulphonamide,
- [882] N-{3-[2-(morpholin-4-yl)ethyl]-1H-indol-5-yl}quinoline-8-sulphonamide,
- [883] N-{3-[2-(morpholin-4-yl)ethyl]-1H-indol-5-yl}-4-phenylbenzenesulphonamide,
- [884] N-[3-(4-methylpiperazin-1-yl)ethyl-1H-indol-5-yl]naphthalene-2-sulphonamide and
- [885] N-[3-(4-methylpiperazin-1-yl)ethyl-1H-indol-5-yl]-5-chloronaphthalene-1-sulphonamide;
and their corresponding salts and solvates.
[0920]The compounds of general formula (Ik) may be prepared according to the disclosure of WO 2004/098588.
[0921]In another embodiment of the present invention compounds of general formula (Ib) are selected from the group consisting of compounds of general formula (Im)
[0000]
[0000]wherein
R1mrepresents a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or a —(CH2)mm—NR13mR14mmoiety with mm=0, 1, 2, 3, 4 or 5;
R2mrepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —CN; —O—R10m; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R3mrepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —CN; —O—R10m; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R4m, R6mand R7m, independently of one another, each represent a hydrogen atom; —NO2; —CN; —O—R10m; a halogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R10mrepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R13mand R14m, independently of one another, each represent a hydrogen atom; or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or
R13mand R14mtogether with the bridging nitrogen form an unsubstituted or at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R15mrepresents a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical or a —S(═O)2—R16mmoiety;
and R16mrepresents an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[0922]Preferred compounds of general formula (Im) are those, wherein
[0000]R1mrepresents a —(CH2)mm—NR13mR14mradical,
R2mrepresents hydrogen or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl and isopropyl, more preferably hydrogen or methyl,
R3m, R4mand R6meach represent hydrogen,
R7mrepresents a hydrogen atom, a chlorine atom, a bromine atom or —O—CH3,
R15mrepresents hydrogen,
R13mand R14m, identical or different, each represent methyl, ethyl, n-propyl or isopropyl, more preferably methyl or ethyl,
or
R13mand R14mtogether with the bridging nitrogen form a 5- or 6-membered heterocyclic ring, more preferably form pyrrolidine or piperidine,
R16mrepresents an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, quinolinyl, benzo[b]furanyl, benzo[b]thiophenyl, benzo[1,2,5]thiadiazolyl, thiophenyl and imidazo[2,1-b]thiazolyl which may be substituted by 1, 2 or 3 substituents selected from the group consisting of fluorine, bromine, chlorine, methyl, phenyl, nitro, —C(═O)—CH3, —O—CH3and —O-phenyl and/or which may be bonded via a C1-2alkylene group or a C2alkenylene group,
and
mm is 2 or 3,
optionally in form of one of its stereoisomers, preferably enantiomers or diastereomers, its racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers or diastereomers, in any mixing ratio, or a salt thereof, preferably a corresponding, physiologically acceptable salt thereof, or a corresponding solvate thereof.
[0923]Particularly preferred compounds of general formula (Im) are those selected from the group consisting of:
- [886] N-[1-(2-dimethylamino ethyl)-1H-indole-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulfonamide,
- [887] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-naphthalene-2-sulfonamide,
- [888] N-[1-(2-dimethylamino ethyl)-1H-indole-5-yl]-naphthalene-1-sulfonamide,
- [889] N-[1-(2-dimethylamino ethyl)-1H-indole-5-yl]-5-chloronaphthalene-1-sulfonamide,
- [890] N-[1-(2-dimethylamino ethyl)-1H-indole-5-yl]-benzenesulfonamide,
- [891] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-quinoline-8-sulfonamide,
- [892] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-4-phenoxybenzenesulfonamide,
- [893] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-4-methylbenzenesulfonamide,
- [894] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-5-chlorothiophene-2-sulfonamide,
- [895] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-benzo[1,2,5]thiadiazole-4-sulfonamide,
- [896] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-6-chloroimidazo[2,1-b]thiazole-5-sulfonamide,
- [897] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-3,5-dichlorobenzenesulfonamide,
- [898] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-3-bromobenzenesulfonamide,
- [899] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-3-nitrobenzenesulfonamide,
- [900] N-[1-(2-dimethylamino ethyl)-1H-indole-5-yl]-1-phenylmethanesulfonamide,
- [901] N-[1-(2-pyrrolidine-1-yl-ethyl)-1H-indole-5-yl]-naphthalene-2-sulfonamide,
- [902] N-[1-(2-pyrrolidine-1-yl-ethyl)-1H-indole-5-yl]-naphthalene-1-sulfonamide,
- [903] N-[1-(2-pyrrolidine-1-yl-ethyl)-1H-indole-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulfonamide,
- [904] trans-N-[1-(2-dimethylamino ethyl)-1H-indole-5-yl]-2-phenylethenesulfonamide,
- [905] N-[1-(2-dimethylamino ethyl)-1H-indole-5-yl]-4,5-dichlorothiophene-2-sulfonamide,
- [906] N-[1-(2-dimethylamino ethyl)-1H-indole-5-yl]-4-acetylbenzenesulfonamide,
- [907] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-4-bromobenzenesulfonamide,
- [908] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-4-methoxybenzenesulfonamide,
- [909] N-[3-(2-diethylamino ethyl)-1H-indole-5-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulfonamide,
- [910] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-4-nitrobenzenesulfonamide,
- [911] N-[1-(2-dimethylaminoethyl)-1H-indole-5-yl]-4-fluorobenzenesulfonamide,
- [912] N-[1-(2-diethylaminoethyl)-1H-indole-5-yl]-6-chloroimidazo[2,1-b]thiazole-5-sulfonamide,
- [913] N-[1-(2-pyrrolidine-1-yl-ethyl)-1H-indole-5-yl]-]-6-chloroimidazo[2,1-b]thiazole-5-sulfonamide,
- [914] N-(1-(2-(diethylamino)ethyl)-1H-indol-5-yl)-naphthalene-2-sulfonamide,
- [915] N-(1-(2-(diethylamino)ethyl)-1H-indol-5-yl)-naphthalene-1-sulfonamide,
- [916] N-(1-(2-(diethylamino)ethyl)-1H-indol-5-yl)-4-phenylbenzenesulfonamide,
- [917] 5-chloro-N-(1-(2-(dimethylamino)ethyl)-2-methyl-1H-indol-5-yl)-3-methylbenzo[b]thiophene-2-sulfonamide,
- [918] N-(1-(2-(dimethylamino)ethyl)-2-methyl-1H-indol-5-yl)-naphthalene-2-sulfonamide,
- [919] N-(1-(2-(dimethylamino)ethyl)-2-methyl-1H-indol-5-yl)-naphthalene-1-sulfonamide,
- [920] 6-chloro-N-(1-(2-(dimethylamino)ethyl)-2-methyl-1H-indol-5-yl)imidazo[2,1-b]thiazole-5-sulfonamide,
- [921] N-(1-(2-(dimethylamino)ethyl)-2-methyl-1H-indol-5-yl)-4-phenylbenzenesulfonamide,
- [922] N-(1-(2-dimethylamino)ethyl)-2-methyl-1H-indol-5-yl)-2-(naphth-1-yl)-ethanesulfonamide,
- [923] N-(1-(2-(dimethylamino)ethyl)-2-methyl-1H-indol-5-yl)-4-phenoxy-benzenesulfonamide,
- [924] 3,5-dichloro-N-(1-(2-(dimethylamino)ethyl)-2-methyl-1H-indol-5-yl)-benzenesulfonamide,
- [925] N-(1-(2-(dimethylamino)ethyl)-2-methyl-1H-indol-5-yl)benzo[b]thiophene-3-sulfonamide,
- [926] N-(1-(2-(diethylamino)ethyl)-1H-indol-5-yl)benzo[b]thiophene-3-sulfonamide
- [927] N-(1-(2-(dimethylamino)ethyl)-1H-indol-5-yl)benzo[b]thiophene-3-sulfonamide,
- [928] 5-chloro-3-methyl-N-(1-(3-(piperidin-1-yl)propyl)-1H-indol-5-yl)benzo[b]thiophene-2-sulfonamide,
- [929] N-(1-(3-(piperidin-1-yl)propyl)-1H-indol-5-yl)naphthalene-2-sulfonamide,
- [930] N-(1-(3-(piperidin-1-yl)propyl)-1H-indol-5-yl)naphthalene-1-sulfonamide,
- [931] 6-chloro-N-(1-(3-piperidin-1-yl)propyl)-1H-indol-5-yl)imidazo[2,1-b]thiazole-5-sulfonamide,
- [932] 4-phenyl-N-(1-(3-(piperidin-1-yl)propyl)-1H-indol-5-yl)benzenesulfonamide,
- [933] 2-(naphth-1-yl)-N-(1-(3-(piperidin-1-yl)propyl)-1H-indol-5-yl)ethanesulfonamide,
- [934] 4-phenoxy-N-(1-(3-(piperidin-1-yl)propyl)-1H-indol-5-yl)benzenesulfonamide,
- [935] 3,5-dichloro-N-(1-(3-(piperidin-1-yl)propyl)-1H-indol-5-yl)benzenesulfonylamide,
- [936] 4,5-dichloro-N-(1-(3-(piperidin-1-yl)propyl)-1H-indol-5-yl)thiophene-2-sulfonamide and
- [937] 5-chloro-N-(1-(3-(piperidin-1-yl)propyl)-1H-indol-5-yl)naphthalene-1-sulfonamide,
- [938] N-(3-(2-(diethylamino)ethyl)-7-methoxy-1H-indol-5-yl)naphthalene-2-sulfonamide,
- [939] N-(3-(2-(diethylamino)ethyl)-7-methoxy-1H-indol-5-yl)benzo[c][1,2,5]thiadiazole-4-sulfonamide,
- [940] 6-chloro-N-(3-(2-(diethylamino)ethyl)-7-methoxy-1H-indol-5-yl)imidazo[2,1-b]thiazole-5-sulfonamide,
and their corresponding salts and solvates.
[0979]The compounds of general formula (Im) may be prepared according to the disclosure of WO 2005/013977 and WO 2006/024535.
[0980]In another embodiment of the present invention compounds of general formula (Ib) are selected from the group consisting of compounds of general formula (In)
[0000]
[0000]wherein
R1nrepresents a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or a —(CH2)mn—NR13nR14nmoiety with mn=0, 1, 2, 3, 4 or 5;
R2nrepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —CN; —O—R10n; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R3nrepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —CN; —O—R10n; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or a —(CH2)nn—NR13nR14nmoiety with nn=0, 1, 2, 3, 4 or 5;
R5n, R6nand R7n, independently of one another, each represent a hydrogen atom; —NO2; —CN; —O—R10n; a halogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R10nrepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R13nand R14n, independently of one another, each represent a hydrogen atom; or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or
R13nand R14ntogether with the bridging nitrogen form an unsubstituted or at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R15nrepresents a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical or a —S(═O)2—R16nmoiety;
R16nrepresents an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[0981]Preferred compounds of general formula (In) are those, wherein
[0000]R1nrepresents a hydrogen atom or a —(CH2)mn—NR13nR14nradical,
R2n, R5n, R6nand R7neach represent hydrogen,
R3nrepresents a hydrogen atom or a —(CH2)nn—NR13nR14nmoiety with nn=0, 1 or 2;
R15nrepresents hydrogen,
R13nand R14n, identical or different, each represent methyl, ethyl, n-propyl, isopropyl, more preferably methyl,
or
R13nand R14ntogether with the bridging nitrogen form a 5- or 6-membered heterocyclic ring, more preferably form pyrrolidine or piperidine,
and
R16nrepresents an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, benzo[b]thiophenyl and imidazo[2,1-b]thiazolyl which may be substituted by 1, 2 or 3 substituents selected from the group consisting of chlorine, methyl, phenyl and —O-phenyl and/or which may be bonded via a C1-2alkylene group, and
mn is 1 or 2;
optionally in form of one of its stereoisomers, preferably enantiomers or diastereomers, its racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers or diastereomers, in any mixing ratio, or a salt thereof, preferably a corresponding, physiologically acceptable salt thereof, or a corresponding solvate thereof.
[0982]Particularly preferred compounds of general formula (In) are those selected from the group consisting of:
- [941] N-[1-(2-dimethylaminoethyl)-1H-indole-4-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulfonamide,
- [942] N-[1-(2-dimethylaminoethyl)-1H-indole-4-yl]-naphtalene-2-sulfonamide,
- [943] N-[1-(2-dimethylaminoethyl)-1H-indole-4-yl]-naphtalene-1-sulfonamide,
- [944] N-[1-(2-dimethylaminoethyl)-1H-indole-4-yl]-4-phenylbenzenesulfonamide,
- [945] N-[1-(2-dimethylaminoethyl)-1H-indole-4-yl]-2-(naphtalene-1-yl)-ethanesulfonamide,
- [946] N-[1-(2-dimethylaminoethyl)-1H-indole-4-yl]-4-phenoxybenzenesulfonamide,
- [947] N-[1-(2-dimethylaminoethyl)-1H-indole-4-yl]-3,5-dichlorobenzenesulfonamide and
- [948] 6-chloro-N-[1-(2-dimethylaminoethyl)-1H-indol-4-yl]-imidazo[2,1-b]thiazole-5-sulfonamide
- [949] N-(3-(2-(dimethylamino)ethyl)-1H-indol-4-yl)-4-biphenylsulfonamide,
- [950] N-(3-(2-(dimethylamino)ethyl)-1H-indol-4-yl)-4-phenoxybenzenesulfonamide,
- [951] 3,5-dichloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-4-yl)benzenesulfonamide,
- [952] 5-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-4-yl)-3-methylbenzo[b]thiophene-2-sulfonamide,
- [953] N-(3-(2-(dimethylamino)ethyl)-1H-indol-4-yl)naphthalene-1-sulfonamide,
- [954] 5-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-4-yl)naphthalene-2-sulfonamide,
- [955] N-(3-(2-(dimethylamino)ethyl)-1H-indol-4-yl)naphthalene-2-sulfonamide,
- [956] 6-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-4-yl)imidazo[2,1-b]thiazole-5-sulfonamide,
- [957] N-(3-(2-(dimethylamino)ethyl)-1H-indol-4-yl)-2-(naphthalen-1-yl)ethanesulfonamide,
and their corresponding salts and solvates.
[1000]The compounds of general formula (In) may be prepared according to the disclosure of WO 2005/13978 and WO 2006/024535.
[1001]In another embodiment of the present invention compounds of general formula (Ib) are selected from the group consisting of compounds of general formula (Io)
[0000]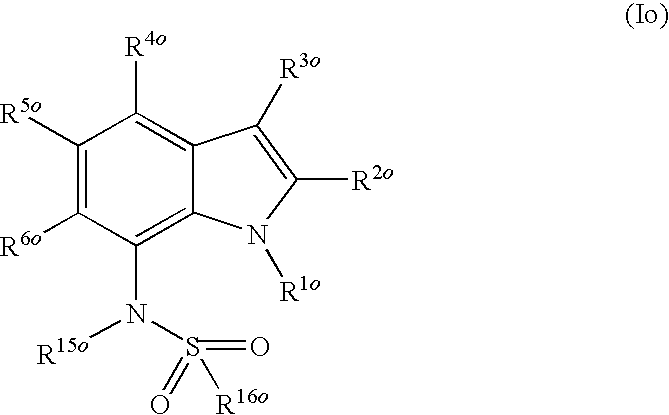
[0000]wherein
R1orepresents a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or a —(CH2)mo—NR13oR14omoiety with mo=0, 1, 2, 3, 4 or 5;
R2orepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —CN; —O—R10o; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R3orepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —CN; —O—R10o; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; a —CH(OC2H5)—CH2—NR13oR14omoiety or a —(CH2)no—NR13oR14omoiety with no=0, 1, 2, 3, 4 or 5;
R4o, R5oand R6o, independently of one another, each represent a hydrogen atom; —NO2; —CN; —O—R10o; a halogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R10orepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R13oand R14o, independently of one another, each represent a hydrogen atom; or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or
R13oand R14otogether with the bridging nitrogen form an unsubstituted or at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R15orepresents a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical or a —S(═O)2—R16omoiety;
and R16orepresents an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1002]Preferred compounds of general formula (Io) are those, wherein
[0000]R1is —(CH2)mo—NR13oR14oradical,
R2o, R4oand R6oeach represent hydrogen,
R3orepresents a hydrogen atom, a —CH(OC2H5)—CH2—NR13oR14omoiety or a —(CH2)no—NR13oR14omoiety with no=0, 1 or 2,
R5orepresents a hydrogen atom, chlorine or bromine,
R15orepresents hydrogen or a —S(═O)2—R16omoiety,
R13oand R14o, identical or different, each represent methyl, ethyl, n-propyl or isopropyl, more preferably methyl,
or
R13oand R14otogether with the bridging nitrogen atom form a 5- or 6-membered heterocyclic ring, more preferably form a pyrrolidine or piperidine ring,
R16orepresents an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, benzo[b]thiophenyl and imidazo[2,1-b]thiazolyl which may be substituted by 1, 2 or 3 substituents selected from the group consisting of chlorine, methyl and phenyl and/or which may be bonded via a C1-2alkylene group,
and
no is 1 or 2;
optionally in form of one of its stereoisomers, preferably enantiomers or diastereomers, its racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers or diastereomers, in any mixing ratio, or a salt thereof, preferably a corresponding, physiologically acceptable salt thereof, or a corresponding solvate thereof.
[1003]Particularly preferred compounds of general formula (Io) are those selected from the group consisting of:
- [958] N-[1-(2-dimethylamino ethyl)-1H-indole-7-yl]-naphtalene-1-sulfonamide,
- [959] N-[1-(2-dimethylaminoethyl)-1H-indole-7-yl]-5-chloro-3-methylbenzo[b]thiophene-2-sulfonamide,
- [960] N-[1-(2-dimethylaminoethyl)-1H-indole-7-yl]-4-phenylbenzenesulfonamide and
- [961] N-[1-(2-dimethylaminoethyl)-1H-indole-7-yl]-6-chloroimidazo[2,1-b]thiazole-5-sulfonamide
- [962] 5-chloro-3-methyl-N-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indol-7-yl)-benzo[b]thiophen-2-sulfonamide,
- [963] N-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indol-7-yl)naphthalene-1-sulfonamide,
- [964] 6-chloro-N-(1-(2-(pyrroldin-1-yl)ethyl)-1H-indol-7-yl)imidazo[2,1-b]thiazole-5-sulfonamide and
- [965] 2-(naphth-1-yl)-N-(1-(2-(pyrrolidin-1-yl)ethyl)-1H-indol-7-yl)ethansulfonamide
- [966] 5-chloro-N-(3-(2-(dimethylamino)-1-ethoxyethyl)-1H-indol-7-yl)-3-methylbenzo[b]thiophene-2-sulfonamide,
- [967] 5-chloro-N-(3-(2-(dimethylamino)ethyl)-1H-indol-7-yl)-3-methylbenzo[b]thiophene-2-sulfonamide,
- [968] 7-bis(5-chloro-3-methylbenzo[b]thiophene-2-sulfonyl)amino-3-(2-(diethylamino)-1-ethoxyethyl)-1H-indole,
- [969] 5-chloro-N-(3-(2-(diethylamino)-1-ethoxyethyl)-1H-indol-7-yl)-3-methylbenzo[b]thiophene-2-sulfonamide,
- [970] 7-bis(5-chloro-3-methylbenzo[b]thiophene-2-sulfonyl)amino-3-(2-(dimethylamino)ethyl)-1H-indole,
- [971] 7-bis(5-chloro-3-methylbenzo[b]thiophene-2-sulfonyl)amino-3-(2-(diethylamino)ethyl)-1H-indole,
- [972] 5-chloro-N-(3-(2-(diethylamino)ethyl)-1H-indol-7-yl)-3-methylbenzo[b]thiophene-2-sulfonamide,
- [973] 7-bis(6-chloroimidazo[2,1-b]thiazol-5-ylsulfonyl)amino-3-(2-(dimethylamino)ethyl)-1H-indole,
- [974] N-(5-bromo-3-(2-(pyrrolidin-1-yl)ethyl)-1H-indol-7-yl)-6-chloroimidazo[2,1-b]thiazole-5-sulfonamide,
and their corresponding salts and solvates.
[1021]The compounds of general formula (Io) may be prepared according to the disclosure of WO 2005/13979 and WO 2006/024535.
[1022]In another embodiment of the present invention compounds of general formula (Ib) are selected from the group consisting of compounds of general formula (Ip)
[0000]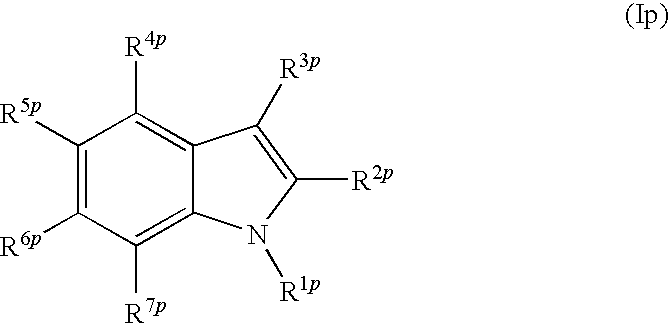
[0000]wherein
R1prepresents a —S(═O)2—R9pmoiety or a —S(═O)2—C(H)ApBpmoiety;
R2prepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —OH; —CN; —O—R10p; —S—R11p; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R3prepresents a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via an unsubstituted or at least mono-substituted alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or a —(CH2)np—NR13pR14pmoiety with np=0, 1, 2, 3, 4 or 5;
R4p, R5p, R6pand R7p, independently of one another, each represent a hydrogen atom; —NO2; —NH2; —OH; —CN; —C(═O)—R8p; —O—R10p; —S—R11p; —NH—R17p; —NR18pR19p; a halogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group;
R8prepresents a hydrogen atom or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
R8p, R17p, R18pand R19p, independently of one another, each represent a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group;
R9prepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
R10pand R11p, independently of one another, each represent a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R13pand R14p, independently of one another, each represent a hydrogen atom; or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or
R13pand R14ptogether with the bridging nitrogen form an unsubstituted or at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
Apand Bptogether with the bridging carbon form an unsubstituted or at least mono-substituted, saturated or unsaturated cycloaliphatic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1023]Preferred compounds of general formula (Ip) are those, wherein
[0000]R1prepresents a —S(═O)2—C(H)ApBpmoiety;
R2p, R3p, R4pand R6peach represent hydrogen,
R3prepresents a —(CH2)np—NR13pR14pmoiety or an unsaturated, optionally at least one nitrogen atom as a ring member containing 5- or 6-membered cycloaliphatic radical, which may be substituted by a methyl group and/or which may be condensed with a 5-membered cycloaliphatic ring,
more preferably R3prepresents a —(CH2)np—NR13pR14pmoiety or a moiety selected from the group consisting of
[0000]
[0000]R5prepresents H, fluorine, chlorine, nitro or a —NH2group,
R13pand R14p, identical or different, each represent methyl, ethyl, n-propyl or isopropyl, more preferably methyl,
or
R13pand R14ptogether with the bridging nitrogen atom form a 5- or 6-membered heterocyclic ring, more preferably form a pyrrolidine or piperidine ring,
Apand Bptogether with the carbon atom to which they are bonded form a saturated or unsaturated C3-C8cycloaliphatic ring, more preferably form a cyclohexyl ring,
and
np is 0, 1 or 2;
optionally in form of one of their stereoisomers, preferably enantiomers or diastereomers, their racemate or in form of a mixture of at least two of their stereoisomers, preferably enantiomers or diastereomers, in any mixing ratio, or a salt thereof, preferably a corresponding physiologically acceptable salt thereof or a corresponding solvate thereof.
[1024]Particularly preferred compounds of general formula (Ip) are those selected from the group consisting of:
- [975] 1-Cyclohexanesulfonyl-3-(1-methyl-1,2,3,6-tetrahydropyridine-4-yl)-5-nitro-1H-indole,
- [976] 5-Chloro-1-cyclohexanesulfonyl-3-(1-methyl-1,2,3,6-tetrahydropyridine-4-yl)-1H-indole,
- [977] 5-Amino-1-cyclohexanesulfonyl-3-(1-methyl-1,2,3,6-tetrahydropyridine-4-yl)-1H-indole and
- [978] 1-Cyclohexanesulfonyl-5-fluoro-3-(1,2,3,5,8,8a-hexahydro-indolizine-7-yl)-1H-indole hydrochloride
and their corresponding salts and solvates.
[1029]The compounds of general formula (Ip) may be prepared according to the disclosure of WO 2005/013974.
[1030]In another embodiment of the present invention compounds of general formula (Ib) are selected from the group consisting of compounds of general formula (Iq)
[0000]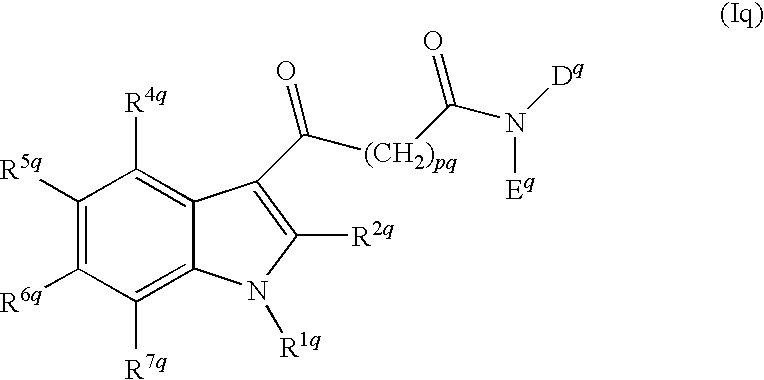
[0000]wherein
R1qrepresents a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical,
which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; a —C(═O)—R8qmoiety; a —S(═O)2—R9qmoiety;
R2qrepresents a hydrogen atom; —F; —Cl; —Br; —I; —NO2; —NH2; —SH; —OH; —CN; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a chain member containing aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R4q, R5q, R6qand R7q, independently of one another, each represent a hydrogen atom; —NO2; —NH2; —SH; —OH; —CN; —C(═O)—OH; —C(═O)—H; —S(═O)2—OH; —C(═O)—NH2; —S(═O)2—NH2; —C(═O)—R8q; —S(═O)2—R9q; —O—R10q; —S—R11q; —C(═O)—OR12q; —N(R15q)—S(═O)2—R16q; —NH—R17q; —NR18qR19q; —C(═O)—NHR20q, —C(═O)—NR21qR22q; —S(═O)2—NHR23q; —S(═O)2—NR24qR25q; —O—C(═O)—R26q; —NH—C(═O)—R27q; —NR28q—C(═O)—R29q; NH—C(═O)—O—R30q; NR31q—C(═O)—O—R32q; —S(═O)2—O—R33q; a halogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
with the proviso that at least one of the substituents R4q, R5q, R6qand R7qrepresents a —N(R15q)—S(═O)2—R16qmoiety;
R8q, R12q, R17q, R18q, R19q, R20q, R21q, R22q, R23q, R24q, R25q, R26q, R27q, R28q, R29q, R30q, R31q, R32qand R33q, independently of one another, each represent a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group;
R9qrepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R10qand R11q, independently of one another, each represent a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene group;
R15qrepresents a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical or a —S(═O)2—R16qmoiety;
R16qrepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
Dqand Eqtogether with the bridging nitrogen form an unsubstituted or at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
or
Dqand Eq, independently of one another, each represent a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1031]Preferred compounds of general formula (Iq) are those, wherein
[0000]pq is 0,
R1qrepresents a hydrogen atom,
R2qrepresents a hydrogen atom,
Dqand Eq, identical or different, represent an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl,
one of the substituents R4q, R5q, R6qand R7qrepresents an —N(R15q)—S(═O)—R16q-moiety while the other three of these substituents each represent a hydrogen atom,
R15qrepresents a hydrogen atom,
R16qrepresents an aryl or heteroaryl radical selected from the group consisting of phenyl, 1-naphthyl, 2-naphthyl, pyrazolyl, thiophenyl (thiophenyl), benzo[b]-thiophenyl, benzo[b]furanyl, quinolinyl, isoquinolinyl, imidazo[2,1-b]thiazolyl, 2-oxo-2,3-dihydro-benzooxazolyl and 2-oxo-2,3-dihydrobenzo[d]thiazolyl, whereby said aryl or heteroaryl radical may be bonded via a —(CH2)1, 2 or 3— group and/or may be substituted by 1, 2, 3, 4 or 5 substituents independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, F, Cl, Br, I, —CN, —CF3, —CF2H, CFH2, —C(═O)—O—CH3, C(═O)—O—CH2—CH3, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, phenyl, phenoxy and benzyl;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1032]Particularly preferred compounds of general formula (Iq) are those selected from the group consisting of:
- [979] 2-[5-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonylamino)-1H-indol-3-yl]-N,N-diethyl-2-oxoacetamide,
- [980] N,N-Diethyl-2-[5-(naphthalene-2-sulfonylamino)-1H-indol-3-yl]-2-oxo-acetamide,
- [981] N,N-Diethyl-2-[5-(naphthalene-1-sulfonylamino)-1H-indol-3-yl]-2-oxo-acetamide,
- [982] 2-[5-(Biphenyl-4-sulfonylamino)-1H-indol-3-yl]-N,N-diethyl-2-oxo-acetamide,
- [983] N,N-Diethyl-2-oxo-2-[5-(quinoline-8-sulfonylamino)-1H-indol-3-yl]-acetamide,
- [984] N,N-Dimethyl-2-[5-(naphthalene-2-sulfonylamino)-1H-indol-3-yl]-2-oxo-acetamide,
- [985] N,N-Dimethyl-2-[5-(naphthalene-1-sulfonylamino)-1H-indol-3-yl]-2-oxo-acetamide,
- [986] 2-[5-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonylamino)-1H-indol-3-yl]-N,N-dimethyl-2-oxo-acetamide,
- [987] 2-[5-(6-Chloro-imidazo[2,1-b]thiazole-5-sulfonylamino)-1H-indol-3-yl]-N,N-diethyl-2-oxo-acetamide,
- [988] 2-[5-(6-Chloro-imidazo[2,1-b]thiazole-5-sulfonylamino)-1H-indol-3-yl]-N,N-dimethyl-2-oxo-acetamide,
- [989] N,N-Dimethyl-2-[4-(naphthalene-1-sulfonylamino)-1H-indol-3-yl]-2-oxo-acetamide,
- [990] 2-[4-(5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonylamino)-1H-indole-3-yl]-N,N-dimethyl-2-oxo-acetamide,
- [991] 2-[4-(6-Chloro-imidazo[2,1-b]thiazole-5-sulfonylamino)-1H-indol-3-yl]-N,N-dimethyl-2-oxo-acetamide,
- [992] N,N-Dimethyl-2-[5-[(4-fluoro-3-methyl-phenyl)-1-sulfonylamino]-1H-indol-3-yl]-2-oxo-acetamide,
- [993] 5-(3-Dimethylaminooxalyl-1H-indol-5-ylsulfamoyl)-3-methyl-benzofuran-2-carboxylic acid ethyl ester,
- [994] 2-[5-(Biphenyl-4-sulfonylamino)-1H-indol-3-yl]-N,N-dimethyl-2-oxo-acetamide,
- [995] N,N-Dimethyl-2-oxo-2-[5-(2-oxo-2,3-dihydro-benzoxazole-6-sulfonylamino)-1H-indol-3-yl]-acetamide,
- [996] N,N-Dimethyl-2-oxo-2-[5-(2-oxo-2,3-dihydrobenzo[d]thiazole-6-sulfonamido)-1H-indol-3-yl]acetamide,
- [997] 2-[5-[(4-Cyclohexyl-phenyl)-1-sulfonylamino]-1H-indol-3-yl]-N,N-dimethyl-2-oxo-acetamide,
- [998] N,N-Dimethyl-2-[5-[(4-phenoxy-phenyl)-1-sulfonylamino]-1H-indol-3-yl]-2-oxo-acetamide,
- [999] 2-(5-(5-chloro-3-methylbenzo[b]thiophene-2-sulfonamido)-2-methyl-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide,
- [1000] 2-(5-(6-chloroimidazo[2,1-b]thiazole-5-sulfonamido)-2-methyl-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide,
- [1001] 2-(6-(5-chloro-3-methylbenzo[b]thiophene-2-sulfonamido)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide,
- [1002] N,N-dimethyl-2-(6-(naphthalene-3-sulfonamido)-1H-indol-3-yl)-2-oxoacetamide
- [1003] 2-(6-(biphenyl-4-sulfonamido)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide,
- [1004] N,N-dimethyl-2-(6-(naphthalene-1-sulfonamido)-1H-indol-3-yl)-2-oxoacetamide,
- [1005] N,N-dimethyl-2-(6-(2-(naphthalen-1-yl)ethylsulfonamido)-1H-indol-3-yl)-2-oxoacetamide,
- [1006] N,N-dimethyl-2-oxo-2-(6-(4-phenoxyphenylsulfonamido)-1H-indol-3-yl)acetamide,
- [1007] 2-(6-(3,4-dichlorothiophene-2-sulfonamido)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide,
- [1008] 2-(6-(3,5-dichlorophenylsulfonamido)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide,
- [1009] 2-(6-(1-chloronaphthalene-6-sulfonamido)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide,
- [1010] 2-(6-(6-chloroimidazo[2,1-b]thiazole-5-sulfonamido)-1H-indol-3-yl)-N,N-dimethyl-2-oxoacetamide,
- [1011] N,N-diethyl-2-(2-methyl-5-(5-methyl-1-phenyl-1H-pyrazole-4-sulfonamido)-1H-indol-3-yl)-2-oxoacetamide and
- [1012] N,N-diethyl-2-(2-methyl-5-(1,3,5-trimethyl-1H-pyrazole-4-sulfonamido)-1H-indol-3-yl)-2-oxoacetamide;
and their corresponding salts and solvates.
[1067]The compounds of general formula (Iq) may be prepared according to the disclosure of WO 2006/015867.
[1068]In another embodiment of the present invention as component (A) at least one compound is present which is selected from the group consisting of indazolyl- and (2,3)-dihydro-indolyl-derived sulfonamide compounds of general formula (Ic)
[0000]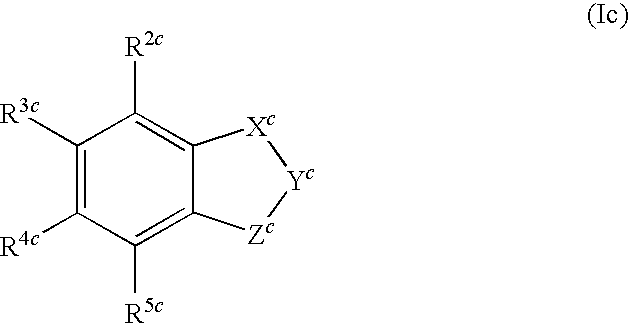
[0000]wherein
Xc—Ycfrom left to right represents CR1c═N and Zcis N[(CH2c)ncR6c]
or
Xc—Ycfrom left to right represents CR7c═N, Zcis NH, R7crepresents the following moiety
[0000]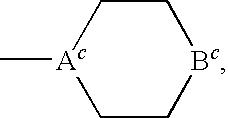
[0000]Acrepresents CH or N and Bcrepresents NR8c, O or S;
Xc—Ycfrom left to right represents C[(CH2c)ncR9c]═N and Zcis NR10c
or
Xc—Ycrepresents CH2—CH2and Zcis N[(CH2c)ncR11c];
nc is 0, 1, 2, 3 or 4;
R1crepresents a hydrogen atom; NO2; —NH2; —SH; —OH; —CN; —C(═O)—R12c; —OR13c; —SR14c; —F; —Cl, —Br; —I; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R2c, R3c, R4cand R5c, independently of one another, each represent a hydrogen atom; —NO2; —NH2; —SH; —OH; —CN; —C(═O)—H; —C(═O)—R12c; —OR13c; —SR14c; —N(R15c)—S(═O)2—R16c; —NH—R17c; —NR18cR19c; —F; —Cl, —Br; —I; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
with the proviso that at least one of the substituents R2c, R3c, R4cand R5crepresents a —N(R15c)—S(═O)2—R16cmoiety;
R6c, R9cand R11c, independently of one another, each represent a —NR20cR21cradical
or
a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R8crepresents —C(═O)—R22c; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R10crepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical-; or a —S(═O)2—R23cmoiety;
R12c, R13c, R14c, R17c, R18cand R19c, independently of one another, each represent a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R15crepresents a hydrogen atom; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or a —S(═O)2—R24cmoiety;
R16cand R24c, independently of one another, each represent an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R20cand R21c, independently of one another, each represent a hydrogen atom; or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or R20cand R21ctogether with the bridging nitrogen form an unsubstituted or at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R22crepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
and
R23crepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1069]Preferred compounds of general formula (Ic) are selected from the group consisting of compounds of general formula (Ir)
[0000]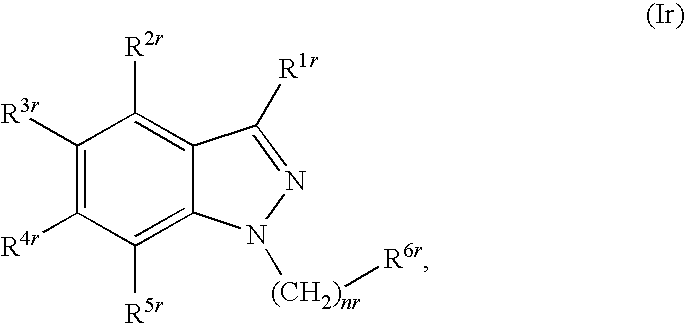
[0000]wherein
nr is 0, 1 or 2;
R1rrepresents a hydrogen atom or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
R2r, R3r, R4rand R5r, independent from one another, each represent a hydrogen atom; —NO2; —NH2; —SH; —OH; —CN; —N(R15r)—S(═O)2—R16r; F; Cl; Br; I; or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
with the proviso that at least one of the substituents R2r, R3r, R4rand R5rrepresents a —N(R15r)—S(═O)2—R16rmoiety;
R6rrepresents a —NR20rR21rradical;
R15rrepresents a hydrogen atom or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl
R16rrepresents an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, benzo[b]furanyl, benzo[b]thiophenyl and imidazo[2,1-b]thiazolyl, which may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, F, Cl, Br, I, —CN, phenyl, phenoxy and benzyl
and
R20rand R21r, independent from one another, each represent an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1070]Particularly preferred compounds of general formula (Ir) are those selected from the group consisting of:
- [1013] N-(1-(2-(Dimethylamino)ethyl)-1H-indazol-6-yl)napthalene-2-sulfonamide and
- [1014] 5-Chloro-N-(1-(2-(dimethylamino)ethyl)-1H-indazol-6-yl)-3-methylbenzo[b]thiophene-2-sulfonamide;
optionally in form of a physiologically acceptable salt thereof, or a corresponding solvate thereof.
[1073]Preferred compounds of general formula (Ic) are selected from the group consisting of compounds of general formula (Is)
[0000]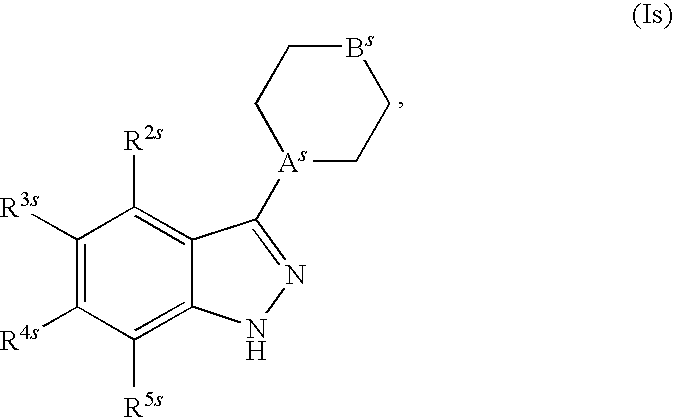
[0000]wherein
Asrepresents CH and Bsrepresents NR8s
or
Asrepresents N and Bsrepresents NR8s
or
Asrepresents N and Bsrepresents O
or
Asrepresents N and Bsrepresents S;
R2s, R3s, R4sand R5s, independent from one another, each represent a hydrogen atom; —NO2; —NH2; —SH; —OH; —CN; —N(R15s)—S(═O)2—R16s; F; Cl; Br; I; or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
with the proviso that at least one of the substituents R2s, R3s, R4sand R5srepresents a —N(R15s)—S(═O)2—R16smoiety;
R8srepresents an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
R15srepresents a hydrogen atom or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl
and
R16srepresents an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, benzo[b]furanyl, benzo[b]thiophenyl and imidazo[2,1-b]thiazolyl, which may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, F, Cl, Br, I, —CN, phenyl, phenoxy and benzyl;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1074]Particularly preferred compounds of general formula (Is) are those selected from the group consisting of:
- [1015] Naphthalene-2-sulfonic acid [3-(1-methyl-piperidin-4-yl)-1H-indazol-5-yl]-amide,
- [1016] 5-Chloro-3-methyl-benzo[b]thiophene-2-sulfonic acid [3-(1-methyl-piperidin-4-yl)-1H-indazol-5-yl]-amide,
- [1017] Naphthalene-1-sulfonic acid [3-(1-methyl-piperidin-4-yl)-1H-indazol-5-yl]-amide,
- [1018] 4-Phenylbenzene-4-sulfonic acid [3-(1-methyl-piperidin-4-yl)-1H-indazol-5-yl]-amide,
- [1019] N-[3-(1-Methyl-piperidin-4-yl)-1H-indazol-5-yl]-4-phenoxy-benzenesulfonamide
and
- [1020] N-[3-(1-Methyl-piperidin-4-yl)-1H-indazol-5-yl]-benzenesulfonamide;
optionally in form of a physiologically acceptable salt thereof, or a corresponding solvate thereof.
[1081]Preferred compounds of general formula (Ic) are selected from the group consisting of compounds of general formula (It)
[0000]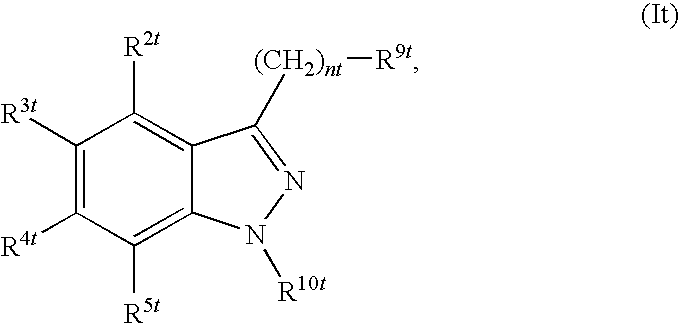
[0000]wherein
nt is 0, 1 or 2;
R2t, R3t, R4tand R5t, independent from one another, each represent a hydrogen atom; —NO2; —NH2; —SH; —OH; —CN; —N(R15t)—S(═O)2—R16t; F; Cl; Br; I; or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
with the proviso that at least one of the substituents R2t, R3t, R4tand R5trepresents a —N(R15t)—S(═O)2—R16tmoiety;
R9trepresents a —NR20tR21tradical;
R10trepresents an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl or a —S(═O)2—R23tmoiety;
R15trepresents a hydrogen atom or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl
R16trepresents an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, benzo[b]furanyl, benzo[b]thiophenyl and imidazo[2,1-b]thiazolyl, which may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, F, Cl, Br, I, —CN, phenyl, phenoxy and benzyl;
R20tand R21t, independent from one another, each represent an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
or
R20tand R21ttogether with the bridging nitrogen atom form an unsubstituted moiety selected from the group consisting of
[0000]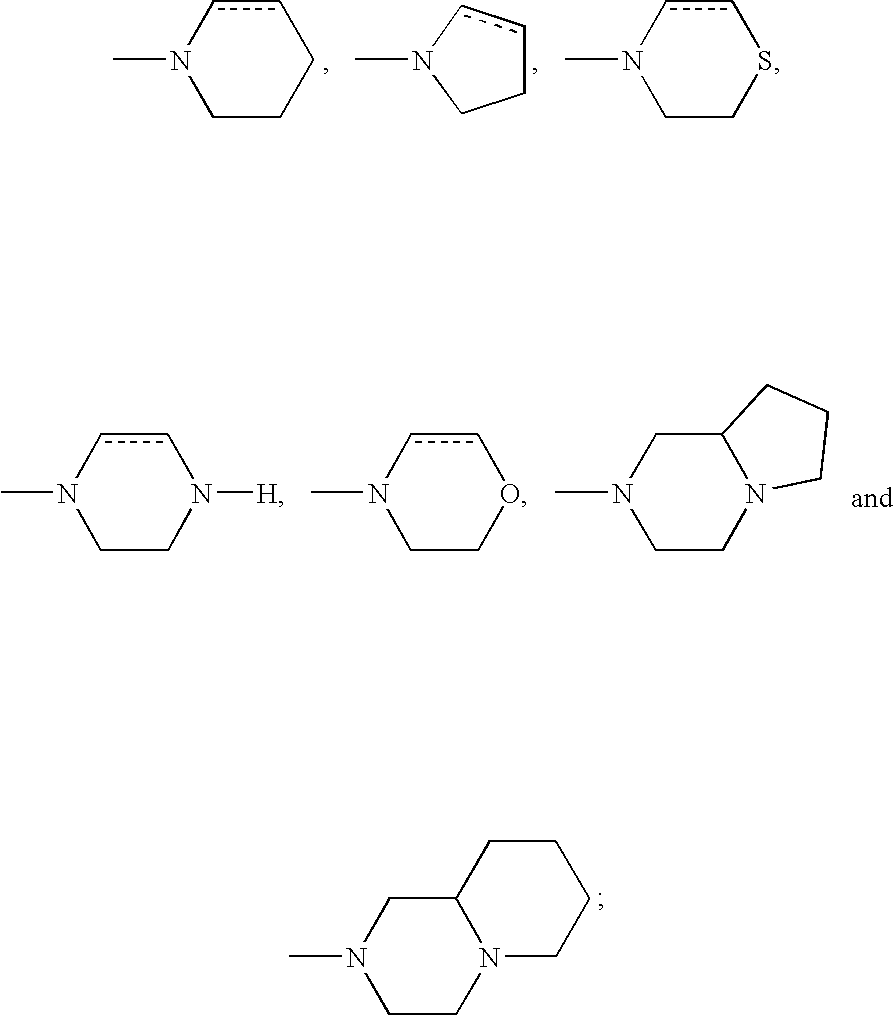
[0000]wherein, if present, the dotted line represents an optional chemical bond;
and
R23trepresents an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl
or an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, benzo[b]furanyl, benzo[b]thiophenyl and imidazo[2,1-b]thiazolyl, which may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, F, Cl, Br, I, —CN, phenyl, phenoxy and benzyl;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1082]Preferred compounds of general formula (Ic) are selected from the group consisting of compounds of general formula (Iu)
[0000]
[0000]wherein
nu is 0, 1 or 2;
R2u, R3u, R4uand R5u, independent from one another, each represent a hydrogen atom; —NO2; —NH2; —SH; —OH; —CN; —C(═O)—H; —C(═O)—R12u; —OR13u; —SR14u; —N(R15u)—S(═O)2—R16u; —NH—R17u; —NR18uR19u; F; Cl; Br; I; or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl which may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of F, Cl, Br, —OH, —NH2, —SH, —O—CH3, —O—C2H5, —NO2, —CN and —S—CH3;
with the proviso that at least one of the substituents R2u, R3u, R4uand R5urepresents a —N(R15u)—S(═O)2—R16umoiety;
R11urepresents a —NR20uR21uradical
or
a (hetero)cycloaliphatic radical selected from the group consisting of
[0000]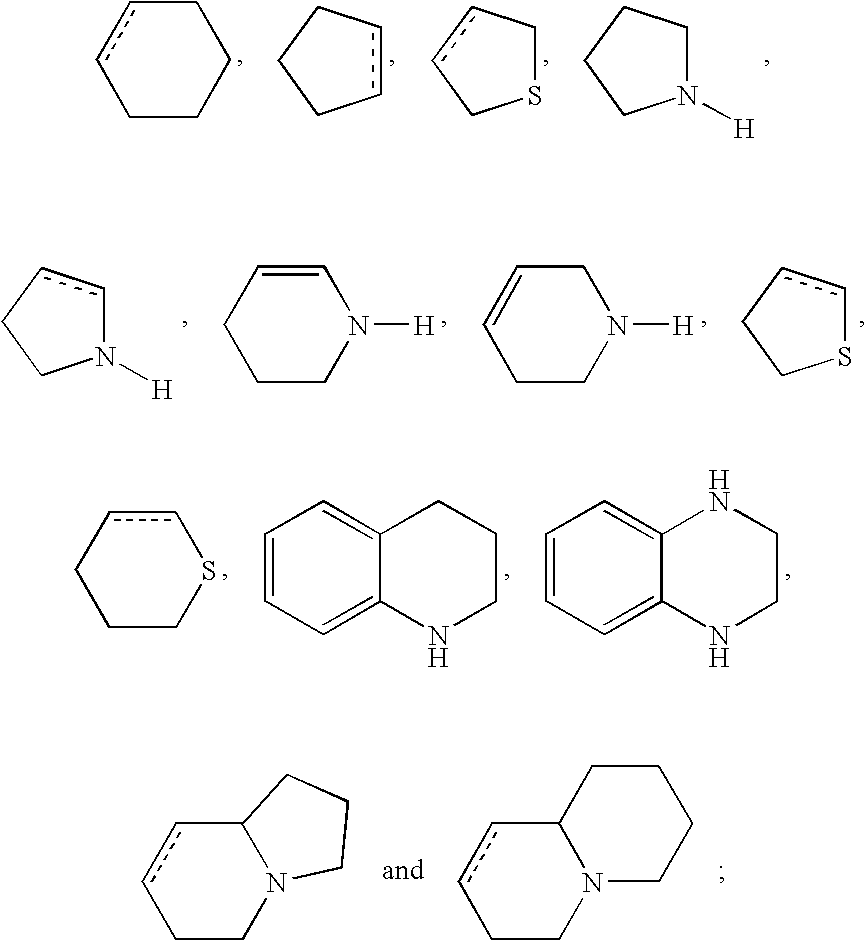
[0000]whereby each of these aforementioned cyclic moieties may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of oxo (═O), thioxo (═S), methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, —O—CH3, —O—C2H5, —S—CH3, —S—C2H5, —C(═O)—OH, —C(═O)—O—CH3, F, Cl, Br, I, —CN, —OCF3, —SCF3, —OH, —SH, —NH2, —NH—CH3, —NH—C2H5, —N(CH3)2, —N(C2H5)2, —NO2, —CHO, —CF2H and —CFH2in any position including the —NH groups and is not bonded via a nitrogen atom and, if present, the dotted line represents an optional chemical bond;
R12u, R13u, R14u, R17u, R18uand R19u, independent from one another, each represent an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl; a (hetero)cycloaliphatic radical selected from the group consisting of cyclopentyl, cyclohexyl, pyrrolidinyl, piperidinyl, morpholinyl, thiomorpholinyl and piperazinyl, which may be bonded via a —(CH2)1, 2 or 3— group and which may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of oxo (═O), thioxo (═S), methyl, ethyl, —O—CH3, —O—C2H5, —S—CH3, —C(═O)—OH, —C(═O)—O—CH3, —F, Cl, Br, I, —CN, —OCF3, —SCF3, —OH, —SH, —NH2, —NH—CH3, —NH—C2H5, —N(CH3)2and —N(C2H5)2; or an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, pyridinyl, furyl (furanyl), thiophenyl (thiophenyl) and pyrrolyl, which may be bonded via a —(CH2)1, 2 or 3— group and which may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of —CF3, methyl, ethyl, —O—CH3, —O—C2H5, —O—CH2—CH2—CH3, —S—CH3, —S—C2H5, —C(═O)—OH, —C(═O)—O—CH3, —C(═O)—O—CH2—CH3, F, Cl, Br, I, —CN, —OCF3, —SCF3, —OH, —SH, —NH2, —NH—CH3, —NH—C2H5, —N(CH3)2and —N(C2H5)2;
R15urepresents a hydrogen atom; or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl which may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of F, Cl, Br, —OH, —NH2, —SH, —O—CH3, —O—C2H5, —NO2, —CN and —S—CH3;
R16urepresents an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, pyridinyl, furyl (furanyl), thiophenyl (thiophenyl), pyrrolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, imidazolyl, pyrazolyl, oxadiazolyl, thiadiazolyl, triazolyl, pyridazinyl, indolyl, isoindolyl, pyrimidinyl, pyrazinyl, quinolinyl, isoquinolinyl, benzo[b]furanyl, benzo[b]thiophenyl, benzothiadiazolyl, benzoxadiazolyl, benzoxazolyl, benzthiazolyl, benzisoxazolyl, benzisothiazolyl and imidazo[2,1-b]thiazolyl, which may be bonded via a —(CH2)1, 2 or 3— group and which may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of —CF3, methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, —O—CH3, —O—C2H5, —S—CH3, —S—C2H5, F, Cl, Br, I, —CN, —OCF3, —SCF3, —OH, —SH, —NH2, —NH—CH3, —NH—C2H5, —N(CH3)2, —N(C2H5)2, —NO2, phenyl, phenoxy and benzyl;
and
R20uand R21u, independent from one another, each represent a hydrogen atom; or an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
or
R20uand R21utogether with the bridging nitrogen atom form a moiety selected from the group consisting of
[0000]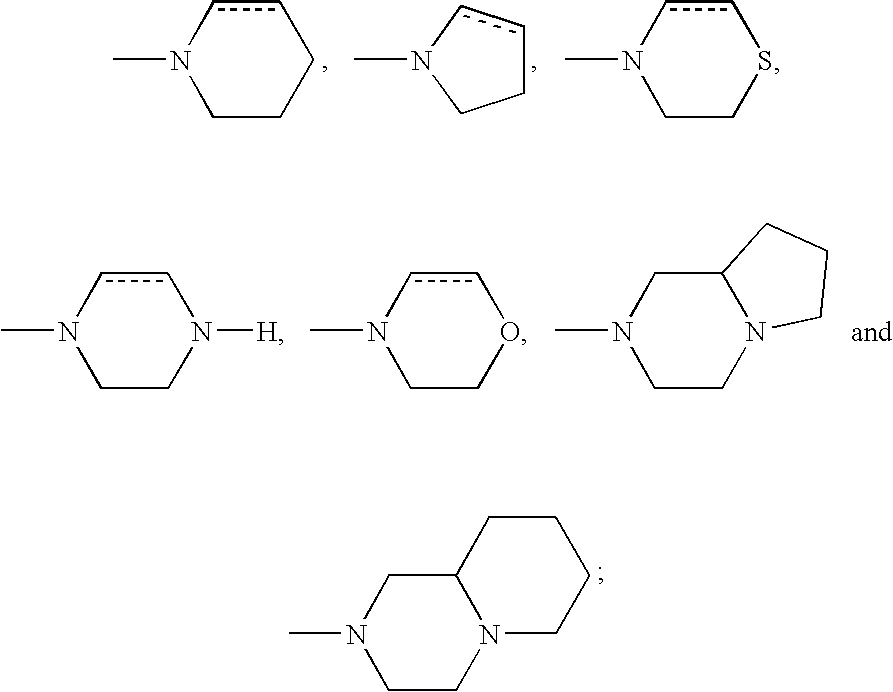
[0000]whereby each of these aforementioned cyclic moieties may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of oxo (═O), thioxo (═S), methyl, ethyl, —O—CH3, —O—C2H5, —S—CH3, —S—C2H5, —C(═O)—OH, —C(═O)—O—CH3, —C(═O)—O—CH2—CH3, F, Cl, Br, I, —CN, —OCF3, —SCF3, —OH, —SH, —NH2, —NH—CH3, —NH—C2H5, —N(CH3)2and —N(C2H5)2in any position including the —NH groups; and, if present, the dotted line represents an optional chemical bond;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof.
[1083]Particularly preferred compounds of general formula (Iu) are those selected from the group consisting of:
- [1021] N-[1-(2-Dimethylamino)ethyl)-2,3-dihydro-1H-indol-6-yl]-6-chloro-imidazo[2,1-b]thiazol-5-sulfonamide;
optionally in form of a physiologically acceptable salt thereof, or a corresponding solvate thereof.
[1085]The compounds of general formulae Ic, Ir, Is, It or Iu given above are prepared by a process, wherein at least one compound of general formula II,
[0000]
[0000]wherein R16chas the meaning given above and X represents a leaving group, preferably a halogen atom, more preferably a chlorine atom, is reacted with at least one compound of general formula III,
[0000]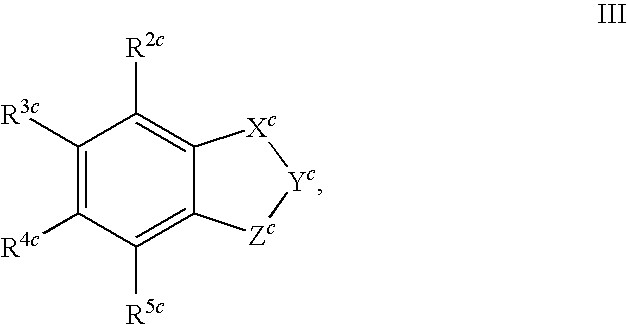
[0000]wherein Xc, Yc, Zcand R2cto R5chave the meaning given above with the proviso that at least one of the substituents R2c, R3c, R4cand R5c, represents a —NH2group, in a suitable reaction medium, preferably in the presence of at least one base, to yield a compound of general formula I, wherein Xc, Yc, Zcand R2cto R5chave the meaning given above with the proviso that at least one of the substituents R2c, R3c, R4cand R5crepresents a —N(H)—S(═O)2—R16cgroup and R16chas the meaning given above, which is optionally purified and/or isolated,
and optionally said compound of general formula I, wherein Xc, Yc, Zcand R2cto R5chave the meaning given above with the proviso that at least one of the substituents R2c, R3c, R4cand R5crepresents a —N(H)—S(═O)2—R16cgroup and R16chas the meaning given above, is reacted with at least one compound of general formula R15c—X, wherein R15chas the meaning given above and X represents a halogen atom, preferably a chlorine atom, in a suitable reaction medium, in the presence of at least one base, preferably at least one base selected from the group consisting of metal hydroxides, metal carbonates, metal alkoxides, preferably sodium methoxide or potassium tert-butoxide, metal hydrides and organometallic compounds, preferably n-butyllithium and tert-butyllithium,
or with at least one compound of general formula X—S(═O)2—R24c, wherein R24chas the meaning given above and X represents a leaving group, preferably a halogen atom, more preferably a chlorine atom, in a suitable reaction medium, preferably in the presence of at least one base,
to yield a compound of general formula I, wherein Xc, Yc, Zcand R2cto R5chave the meaning given above with the proviso that at least one of the substituents R2c, R3c, R4cand Rc5represents a —N(R15c)—S(═O)2—R16cgroup and R15cand R16chave the meaning given above, which is optionally purified and/or isolated.
[1086]Suitable reaction media for the reaction between compounds of general formulae II and III include organic solvents, such as dialkyl ether, preferably diethyl ether, or a cyclic ether, preferably tetrahydrofuran or dioxane; or a halogenated hydrocarbon, preferably dichloromethane or chloroform; an alcohol, preferably methanol or ethanol; a dipolar aprotic solvent, preferably acetonitrile, pyridine or dimethylformamide, or any other suitable reaction medium. Of course, mixtures of at least two classes of solvents or of at least two solvents of one class may also be used.
[1087]The reaction between compounds of general formulae II and III is preferably carried out in the presence of at least one suitable base, for example, an inorganic base such as a hydroxide or a carbonate of an alkali metal and/or an organic base, preferably triethylamine or pyridine.
[1088]The reaction between compounds of general formulae II and III is preferably carried out at a temperature between −10° C. and ambient temperature, i.e. approximately 25° C. and the reaction time is preferably between 5 minutes and 24 hours.
[1089]Suitable reaction media for the reaction between compounds of general formula I, wherein Xc, Yc, Zcand R2cto R5chave the meaning given above with the proviso that at least one of the substituents R2c, R3c, R4cand R5crepresents a —N(H)—S(═O)2—R16cgroup and R16chas the meaning given above and compounds of general formula R15c—X are dialkyl ether, preferably diethyl ether, or a cyclic ether, preferably tetrahydrofuran or dioxane, a hydrocarbon, preferably toluene, an alcohol, preferably methanol or ethanol, a dipolar aprotic solvent, preferably acetonitrile, pyridine or dimethylformamide, or any other suitable reaction medium. Of course, mixtures of at least two classes of solvents or of at least two solvents of one class may also be used.
[1090]The aforementioned reaction is preferably carried out at a temperature between −10° C. and ambient temperature, i.e. approximately 25° C. and the reaction time is preferably 1 and 24 hours.
[1091]Suitable reaction media for the reaction between compounds of general formula I, wherein Xc, Yc, Zcand R2cto R5chave the meaning given above with the proviso that at least one of the substituents R2c, R3c, R4cand R5crepresents a —N(H)—S(═O)2—R16cgroup and R16chas the meaning given above, and compounds of general formula X—S(═O)2—R24cinclude organic solvents, such as dialkyl ether, preferably diethyl ether, or a cyclic ether, preferably tetrahydrofuran or dioxane; or a halogenated hydrocarbon, preferably dichloromethane or chloroform; an alcohol, preferably methanol or ethanol; a dipolar aprotic solvent, preferably acetonitrile, pyridine or dimethylformamide, or any other suitable reaction medium. Of course, mixtures of at least two classes of solvents or of at least two solvents of one class may also be used.
[1092]The aforementioned reaction is preferably carried out in the presence of at least one suitable base, for example, an inorganic base such as a hydroxide or a carbonate of an alkali metal and/or an organic base, preferably triethylamine or pyridine.
[1093]The aforementioned reaction is preferably carried out at a temperature between −10° C. and ambient temperature, i.e. approximately 25° C. and the reaction time is preferably between 5 minutes and 24 hours.
[1094]Those skilled in the art understand that the process described above can also be applied to the synthesis of compounds of general formula Ir, Is, It and Iu given above.
[1095]The compounds of general formula Ic, Ir, Is, It or Iu given above may be purified and/or isolated according to methods well known to those skilled in the art. Preferably, the compounds of general formula Ic, Ir, Is, It or Iu may be isolated by evaporating the reaction medium, addition of water and adjusting the pH value to obtain the compound in form of a solid that can be isolated by filtration, or by extraction with a solvent that is not miscible with water such as chloroform and purification by chromatography or recrystallisation from a suitable solvent.
[1096]The compounds of general formula II are commercially available or may be prepared according to methods well known in the art, for example, analogous to the methods described in the bibliography of E. E. Gilbert, Synthesis, 1969, 1, 3. The respective part of the literature description cited above is hereby incorporated by reference and forms part of the disclosure.
[1097]The compounds of general formula III are commercially available or may also be prepared according to standard methods known in the prior art, for example by methods similar to those described in the literature: Savitskaya, N. V. et al. Synthesis of 5-amino-3-(b-aminoethyl)indazole. Zhurnal Obshchei Khimii (1961), 31 1924-1926; Zhang, Han-Cheng et al. Discovery and Optimization of a Novel Series of Thrombin Receptor (PAR-1) Antagonists: Potent, Selective Peptide Mimetics Based on Indole and Indazole Templates. Journal of Medicinal Chemistry (2001), 44(7), 1021-1024; Ono, Shinichiro et al. Preparation of piperidine derivatives as muscarinic receptors stimulator for treatment of schizophrenia. WO 2004069828 A1; Wrzeciono, U. et al. Synthesis and antiinflammatory activity of some indazole derivatives. Part 36. Azoles. Pharmazie (1993), 48(8); 582-584; Filla, S. A. et al. Preparation of 3-(1-methylpiperidin-4-yl)-1H-indoles and 3-(1-methylpiperidin-4-yl)4-aza-1H-indoles as 5-HT1F agonist. WO 2000/487; Dumas, J. et al. Preparation of bicyclic (hetero)aryl- and pyridine-containing diaryl ureas as Raf kinase and angiogenesis inhibitors useful in the treatment of cancer and other disorders. WO 200478748; Mueller, S. G. et al. Preparation of ethynylpyridines and related compounds as melanin-concentrating hormone receptor (MCH-1) antagonist for the treatment of metabolic disorders. WO 200439780; Maeno, K. Preparation of aminoalkylindazole derivatives as 5-HT2c receptor agonists. WO 98/30548; Zhao, E.-C. et al. Synthesis of dialkylaminoalkyl derivatives of indazole. Zhurnal Obshchei Khimii (1959), 29, 1012-1020. Ham, P. et al. Preparation of N-heteroaryl-4′-oxadiazolylbiphenylcarboxamides as 5HT1D antagonists. WO 9532967A1; Stenkamp, D. et al. Preparation of arylamides as melanin concentrating hormone (MCH) receptor antagonists. WO 200403974.
[1098]The compounds of general formula Ic, Ir, Is, It or Iu given above may be purified and/or isolated according to methods well known to those skilled in the art. Preferably, the compounds of general formula Ic, Ir, Is, It or Iu may be isolated by evaporating the reaction medium, addition of water and then adjusting the pH value to obtain the compound in form of a solid that can be isolated by filtration, or by extraction with a solvent that is not miscible with water such as chloroform and purified by chromatography or recrystallisation from a suitable solvent.
[1099]During some synthetic reactions described above or while preparing the compounds of general formulae Ic, Ir, Is, It, Iu, II and II the protection of sensitive or reactive groups may be necessary and/or desirable. This can be performed by using conventional protective groups like those described in Protective groups in Organic Chemistry, ed. J. F. W. McOmie, Plenum Press, 1973; T. W. Greene & P. G. M. Wuts and Protective Groups in Organic Chemistry, John Wiley & sons, 1991. The respective parts of the description are hereby incorporated by reference and forms part of the disclosure. The protective groups may be eliminated when convenient by means well-known to those skilled in the art.
[1100]If the substituted indazolyl sulfonamide or 2,3-dihydro-indolyl sulfonamide compounds of general formula Ic are obtained in form of a mixture of stereoisomers, particularly enantiomers or diastereomers, said mixtures may be separated by standard procedures known to those skilled in the art, e.g. chromatographic methods or crystallization with chiral reagents.
[1101]The substituted indazolyl sulfonamide or 2,3-dihydro-indolyl sulfonamide compounds of general formula Ic and in each case stereoisomers thereof may be obtained in form of a corresponding salt according to methods well known to those skilled in the art, e.g. by reacting said compound with at least one inorganic and/or organic acid, preferably in a suitable reaction medium. Suitable reaction media include, for example, any of the ones given above. Suitable inorganic acids include but are not limited to hydrochloric acid, hydrobromic acid, phosphoric acid, sulfuric acid, nitric acid, suitable organic acids include but are not limited to citric acid, maleic acid, fumaric acid, tartaric acid, or derivatives thereof, p-toluenesulfonic acid, methanesulfonic acid or camphersulfonic acid.
[1102]In another embodiment of the present invention as component (A) at least one compound is present which is selected from the group consisting of phenyl-piperazine-derived compounds of general formula (Id)
[0000]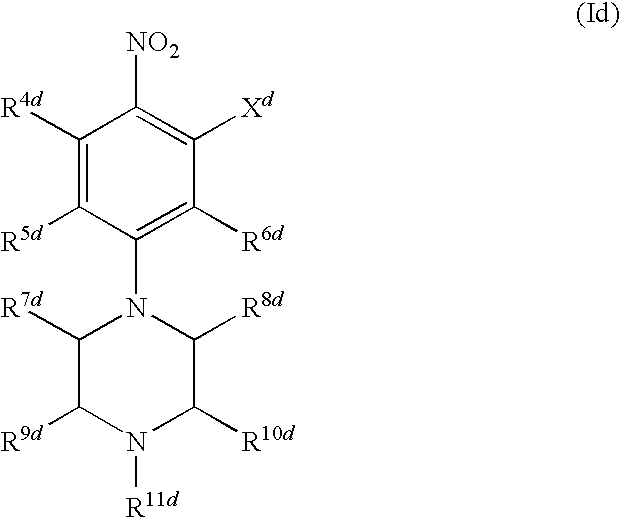
[0000]wherein
Xdrepresents a —NR1dR2dmoiety or a —OR3dmoiety;
R1drepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
an unsubstituted or at least mono-substituted radical selected from the group consisting of adamantyl, bicyclo[2.2.1]heptyl and bicyclo[3.1.1]heptyl, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group which may contain 1, 2 or 3 heteroatom(s) independently selected from the group consisting of nitrogen, oxygen and sulfur as chain member(s);
a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group which may contain 1, 2 or 3 heteroatom(s) independently selected from the group consisting of nitrogen, oxygen and sulfur as chain member(s);
or a —C(═O)—R12dmoiety;
R2drepresents a hydrogen atom or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or
R1dand R2dtogether with the bridging nitrogen form an optionally at least mono-substituted, saturated, unsaturated or aromatic heterocyclic ring which may contain at least one further heteroatom as a ring member and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R3drepresents or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group;
R4d, R5dand R6d, independently of one another, each represent a hydrogen atom or a halogen atom;
or
R4dand R5dtogether with the bridging carbon atoms form an unsubstituted 5- or 6-membered heterocyclic ring which contains 1, 2 or 3 heteroatom(s) independently selected from the group consisting of nitrogen, oxygen and sulfur as ring member(s) and which together with the phenyl ring which it is fused with forms a 9- or 10-membered bicyclic aromatic ring system;
R7dand R8d, independently of one another, each represent a hydrogen atom or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
R9dand R10d, independently of one another, each represent a hydrogen atom or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
R11drepresents a hydrogen atom; or a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical, which may contain 1, 2 or 3 heteroatom(s) independently selected from the group consisting of nitrogen, oxygen and sulfur as chain member(s);
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group;
- a —C(═O)—R13dmoiety or a —S(═O)2—R14dmoiety;
R12drepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group;
and
R13dand R14d, independently of one another, each represent a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group which may contain 1, 2 or 3 heteroatom(s) independently selected from the group consisting of nitrogen, oxygen and sulfur as chain member(s);
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1104]Preferred compounds of general formula (Id) are those, wherein
[0000]Xdrepresents a —NR1dR2dmoiety or a —OR3dmoiety;
R2drepresents an alkyl radical selected from the group consisting of —CH2—CH2—OH and —CH2—CH2—CH2—OH;
an unsubstituted adamantyl radical;
an unsubstituted phenyl or pyrrolyl radical;
an unsubstituted napthyl radical which is bonded via an alkylene group selected form the group consisting of —CH2—, —CH(CH3)—, —CH2—CH2—, —CH2—CH2—CH2— and —CH2—CH2—O—;
a phenyl radical which may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, tert-butyl, methoxy, F and Cl and said phenyl radical is bonded via an alkylene group selected form the group consisting of —CH2—, —CH(CH3)—, —CH(Phenyl)-, —CH2—CH2—, —CH2—CH2—CH2— and —CH2—CH2—O—;
a heteroaryl radical selected from the group consisting of pyridinyl, furanyl and pyrrolyl, whereby said pyridinyl, furanyl or pyrrolyl radical may be substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, tert-butyl, methoxy, F and Cl and said pyridinyl, furanyl or pyrrolyl radical is bonded via an alkylene group selected form the group consisting of —CH2—, —CH(CH3)—, —CH2—CH2—, —CH2—CH2—CH2— and —CH2—CH2—O—;
or a —C(═O)—R12dmoiety;
R2drepresents a hydrogen atom or a methyl radical;
or
R1dand R2dtogether with the bridging nitrogen atom form a moiety selected from the group
consisting of:
[0000]
[0000]R3drepresents an unsubstituted phenyl radical;
R4d, R5dand R6d, identical or different, each represent a hydrogen atom or a fluorine atom;
or
R4dand R5dtogether with the bridging carbon atoms form the following moiety,
[0000]
[0000]which together with the phenyl ring which it is fused with forms the following substituted bicyclic aromatic ring system
[0000]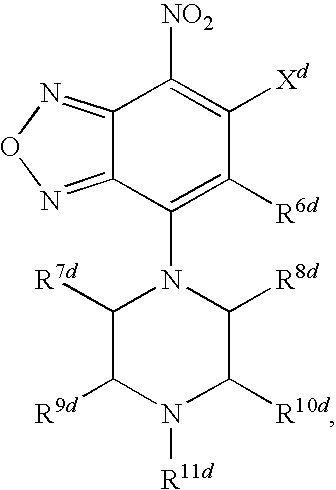
[0000]R7dand R8deach represent a hydrogen atom;
R9dand R10d, identical or different, each represent a hydrogen atom or a methyl radical;
R11drepresents a hydrogen atom;
an alkyl radical selected from the group consisting of methyl, n-butyl and —CH2—CH2—OH;
an unsubstituted phenyl or pyridinyl radical whereby said phenyl or pyridinyl radical may be bonded via a —(CH2)— group;
a —C(═O)—R12dmoiety or a —S(═O)2—R13dmoiety;
R12drepresents a phenyl or a thiophenyl radical whereby said phenyl or thiophenyl radical may be substituted with 1, 2 or 3 substituent(s) selected from the group consisting of methyl and chlorine;
R13drepresents a methyl radical or a phenyl or a thiophenyl radical whereby said phenyl or thiophenyl radical may be substituted with 1, 2 or 3 substituent(s) selected from the group consisting of methyl and chlorine
and
R14drepresents a methyl radical or a phenyl radical which may be substituted with 1, 2 or 3 methyl radical(s);
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof.
[1105]Particularly preferred compounds of general formula (Id) are those selected from the group consisting of:
- [1022] [5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-(2-pyrrol-1-yl-ethyl)-amine,
- [1023] (4-Fluoro-benzyl)-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1024] N-[5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-benzamide,
- [1025] N-Methyl-N-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-benzamide,
- [1026] [5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-pyrrol-1-yl-amine,
- [1027] 2-[5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-2H-pyridazin-3-one,
- [1028] 1-Methyl-4-(4-nitro-3-phenoxy-phenyl)-piperazine,
- [1029] Benzyl-[5-(4-butyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1030] Benzyl-[2-nitro-5-(4-pyridin-2-yl-piperazin-1-yl)-phenyl]-amine and
- [1031] Benzyl-[2-nitro-5-(4-phenyl-2-yl-piperazin-1-yl)-phenyl]-amine;
- [1032] Furan-2-ylmethyl-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1033] 2-[4-(4-Nitro-3-phenethylamino-phenyl)-piperazin-1-yl]-ethanol,
- [1034] (2-Nitro-5-piperazin-1-yl-phenyl)-(2-o-tolyloxy-ethyl)-amine
- [1035] 2-[4-(3-Benzylamino-4-nitro-phenyl)-piperazin-1-yl]-ethanol,
- [1036] 4-[5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-morpholine,
- [1037] 2-[5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-4-phenyl-2H-phthalazin-1-one,
- [1038] [2-(4-Chloro-phenoxy)-ethyl]-[5-(3,5-dimethyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1039] 2-[4-[3-(Benzhydryl-amino)-4-nitro-phenyl]-piperazin-1-yl]-ethanol,
- [1040] 4-[4-Fluoro-5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-morpholine,
- [1041] 2-[2-Nitro-5-[4-(toluene-4-sulfonyl)-piperazin-1-yl]-phenyl]-1,2,3,4-tetrahydro-isoquinoline,
- [1042] 1-[3-(3,5-Dimethyl-pyrazol-1-yl)-4-nitro-phenyl]-4-methyl-piperazine,
- [1043] Benzyl-(2-nitro-5-piperazin-1-yl-phenyl)-amine,
- [1044] [5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-phenethyl-amine,
- [1045] [5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-pyridin-3-ylmethyl-amine,
- [1046] (3-Chloro-phenyl)-[4-[3-[(furanyl-2-ylmethyl)-amino]-4-nitro-phenyl]-piperazin-1-yl]-methanone,
- [1047] (2-Nitro-5-piperazin-1-yl-phenyl)-pyridin-3-ylmethyl-amine,
- [1048] 1-Benzyl-4-(2-nitro-5-piperazin-1-yl-phenyl)-piperazine,
- [1049] Furan-2-ylmethyl-[5-(4-methanesulfonyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1050] Benzhydryl-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1051] (2-Nitro-5-piperazin-1-yl-phenyl)-(2-phenoxy-ethyl)-amine,
- [1052] 2-[5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-4-phenyl-2H-phthalazin-1-one,
- [1053] 1-[3-(3,5-Dimethyl-pyrazol-1-yl)-4-nitro-phenyl]-piperazine,
- [1054] (2-Nitro-5-piperazin-1-yl-phenyl)-phenethyl-amine,
- [1055] [5-(4-Benzenesulfonyl-piperazin-1-yl)-2-nitro-phenyl]-furan-2-ylmethyl-amine,
- [1056] [2-(3,4-Dimethoxy-phenyl)-ethyl]-(2-nitro-5-piperazin-1-yl-phenyl)-amine,
- [1057] [4-[3-[(Furan-2-ylmethyl)-amino]-4-nitro-phenyl]-piperazin-1-yl]-m-tolyl-methanone,
- [1058] [4-[3-[(Furan-2-ylmethyl)-amino]-4-nitro-phenyl]-piperazin-1-yl]-phenyl-methanone,
- [1059] [4-[3-(3,5-Dimethyl-pyrazol-1-yl)-4-nitro-phenyl]-piperazin-1-yl]-thiophen-2-yl-methanone,
- [1060] 4-(4-Ethyl-phenyl)-2-[5-[4-methyl-piperazin-1-yl)-2-nitro-phenyl]-2H-phthalalazin-1-one,
- [1061] 3-[7-(4-Methyl-piperazin-1-yl)-4-nitro-benzo[1,2,5]oxadiazol-5-ylamino]-propan-1-ol,
- [1062] 3-[4-Nitro-7-(4-phenyl-piperazin-1-yl)-benzo[1,2,5]oxadiazol-5-ylamino]-ethan-1-ol,
- [1063] 3-[4-Nitro-7-(4-pyridin-piperazin-1-yl)-benzo[1,2,5]oxadiazol-5-ylamino]-propan-1-ol,
- [1064] [2-(3,4-Dimethoxy-phenyl)-ethyl]-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1065] [2-(3,4-Dimethyl-phenyl)-ethyl]-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1066] [2-(4-tert-Butyl-phenoxy)-ethyl]-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1067] [2-(4-Methoxy-phenoxy)-ethyl]-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1068] [5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-(2-m-tolyloxy-ethyl)-amine,
- [1069] [2-(4-Chloro-phenoxy)-ethyl]-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1070] (2-Nitro-5-piperazin-1-yl-phenyl)-(1-phenyl-ethyl)-amine,
- [1071] [2-(3-Methoxy-phenoxy)-ethyl]-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1072] [2-(2-Methoxy-phenoxy)-ethyl]-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1073] (4-Chloro-benzyl)-(2-nitro-5-piperazin-1-yl-phenyl)-amine,
- [1074] Benzyl-[5-(4-benzyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1075] Benzyl-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1076] [5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-(2-o-tolyloxy-ethyl)-amine,
- [1077] (4-Chloro-benzyl)-[5-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine,
- [1078] Furan-2-ylmethyl-(2-nitro-5-piperazin-1-yl-phenyl)-amine,
- [1079] [2-(4-Chloro-phenoxy)-ethyl]-(2-nitro-5-piperazin-1-yl-phenyl)-amine,
- [1080] [5-(4-Methyl-piperazin-1-yl)-2-nitro-phenyl]-(1-phenyl-ethyl)-amine
- [1081] 1-[4-(3-Benzylamino-4-nitro-phenyl)-piperazin-1-yl]-ethanone,
- [1082] 2-[4-[3-(4-Methylpiperazin-1-yl)-4-nitrophenyl]piperazin-1-yl]ethanol,
- [1083] 2-[4-[3-[2-(Naphthalen-2-yloxy)ethylamino]-4-nitrophenyl]piperazin-1-yl]ethanol,
- [1084] 2-[4-(3-{[1-(1-Adamantyl)ethyl]amino}-4-nitrophenyl)piperazin-1-yl]ethanol
and
- [1085] 2-[4-[3-(3,4-Dimethoxyphenethylamino)-4-nitrophenyl]piperazin-1-yl]ethanol;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof.
[1170]The compounds of general formula Id are prepared by a process, wherein at least one nitrobenzene compound of general formula II,
[0000]
[0000]wherein R4dto R6dhave any of the above given meanings, Ydrepresents a chlorine atom, and Zdrepresents a bromine or iodine atom; is reacted with at least one compound of general formula III,
[0000]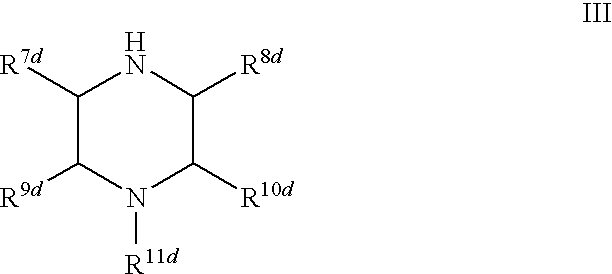
[0000]wherein R7dto R11dhave any of the above given meanings in a suitable reaction medium, preferably in at least an organic solvent, more preferably in at least an organic solvent selected from the group consisting of tetrahydrofuran, toluene and dioxane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium source, even more preferably in the presence of PdCl2(dppf) wherein dppf is 1,1-bis(diphenylphosphino)-ferrocene, and/or at least one auxiliary agent, preferably 1,1-bis(diphenylphosphino)-ferrocene, and/or at least one base, preferably sodium tert-pentoxide, to yield a compound of general formula IV,
[0000]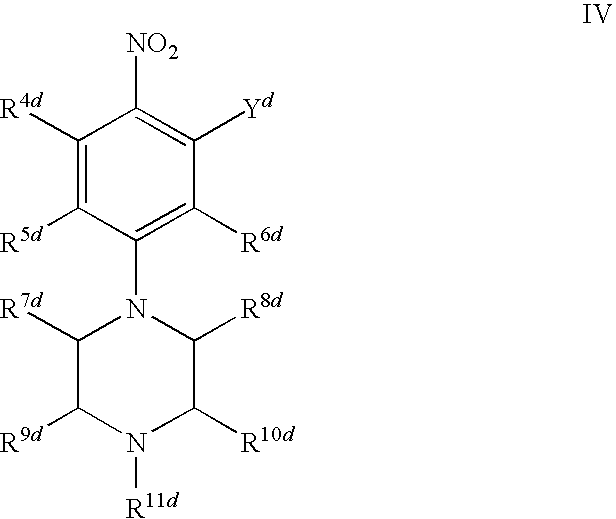
[0000]wherein R4dto R11dhave any of the above given meanings and Ydrepresents a chlorine atom; which is optionally purified and/or isolated, and the compound of general formula IV is reacted with at least one compound of general formula V,
[0000]
[0000]wherein R1dand R2dhave any of the above given meanings, in a suitable reaction medium, preferably in at least an organic solvent, more preferably in at least an organic solvent selected from the group consisting of toluene or dimethoxyethane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium source, even more preferably in the presence of at least a palladium source selected from the group consisting of Pd(OAc)2, wherein OAc is acetate, and Pd2dba3, wherein dba is dibenzylidene acetone, and/or at least one auxiliary agent, preferably (biph)P(tBu)2, wherein biph is biphenyl and tBu is tert-butyl, and/or at least one base, preferably at least one base selected from the group consisting of K3PO4and sodium tert-pentoxide to yield a compound of general formula VI,
[0000]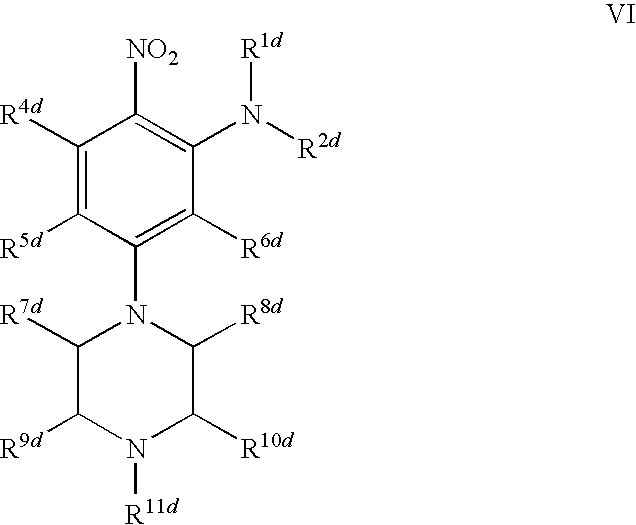
[0000]wherein R1d, R2dand R4dto R11dhave any of the above given meanings which is optionally purified and/or isolated.
[1171]The compounds of general formula Id are prepared by a process, wherein at least one nitrobenzene compound of general formula VII,
[0000]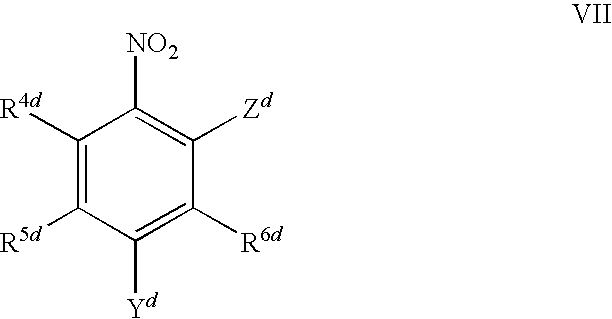
[0000]wherein R4dto R6dhave any of the above given meanings, Zdrepresents a bromine or iodine atom, and Ydrepresents a chlorine atom, is reacted with at least one compound of general formula V,
[0000]
[0000]wherein R1dand R2dhave any of the above given meanings in a suitable reaction medium, preferably in at least an organic solvent, more preferably in at least an organic solvent selected from the group consisting of toluene and dioxane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium and/or copper source, even more preferably in the presence of at least a palladium and/or copper source selected from the group consisting of Pd(OAc)2, wherein OAc is acetate, Pd2dba3, wherein dba is dibenzylidene acetone, and copper(I)iodide, and/or at least one auxiliary agent, preferably at least one auxiliary agent selected from the group consisting of 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (Xantphos), 1,1-bis(diphenylphosphino-ferrocene and P(tBu)3, wherein tBu is tert-Butyl, and/or at least one base, preferably at least one base selected from the group consisting of K3PO4, Cs2CO3and trans-1,2-diamino-methylcyclohexane, to yield a compound of general formula VIII,
[0000]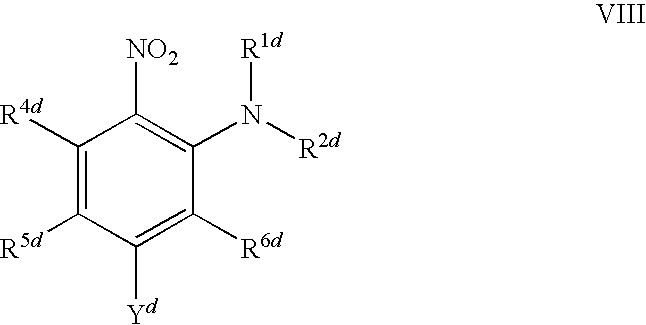
[0000]wherein R1d, R2dand R4dto R6dhave any of the above given meanings and Ydrepresents a chlorine atom; which is optionally purified and/or isolated, and the compound of general formula VIII is reacted with at least one compound of general formula III,
[0000]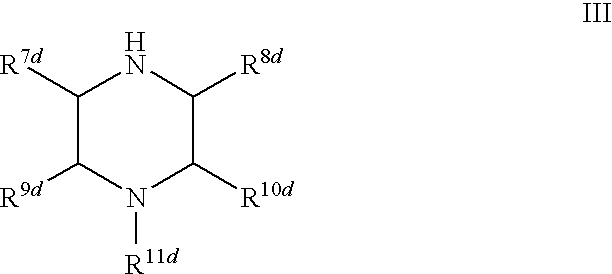
[0000]wherein R7dto R11dhave any of the above given meanings, in a suitable reaction medium, preferably in at least an organic solvent, more preferably in at least an organic solvent selected from the group consisting of toluene, tetrahydrofuran and dimethoxyethane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium source, even more preferably in the presence of at least a palladium source selected from the group consisting of Pd(OAc)2, wherein OAc is acetate, and Pd2dba3, wherein dba is dibenzylidene acetone, and/or at least one auxiliary agent, preferably (biph)P(tBu)2, wherein biph is biphenyl and tBu is tert-butyl, and/or at least one base, preferably at least one base selected from the group consisting of K3PO4or sodium tert-pentoxide, to yield a compound of general formula VI,
[0000]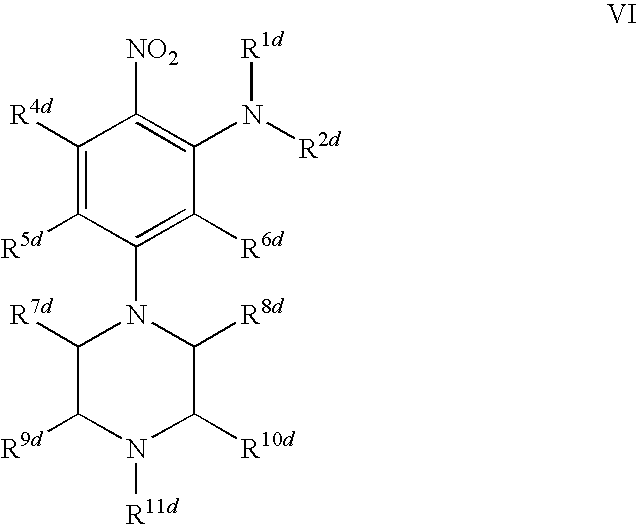
[0000]wherein R1d, R2dand R4dto R11dhave any of the above given meanings, which is optionally purified and/or isolated.
[1172]The compounds of general formula Id are prepared by a process, wherein at least one nitrobenzene compound of general formula II,
[0000]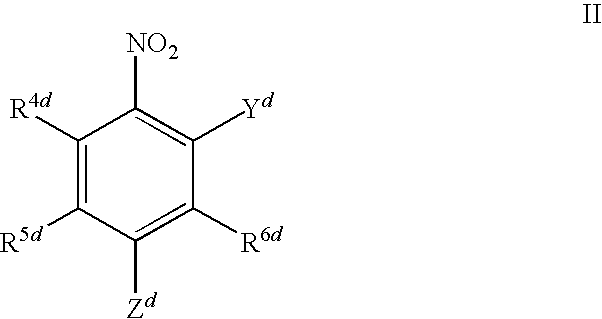
[0000]wherein R4dto R6dhave any of the above given meanings, Ydrepresents a chlorine atom, and Zdrepresents a bromine or iodine atom; is reacted with at least one compound of general formula III,
[0000]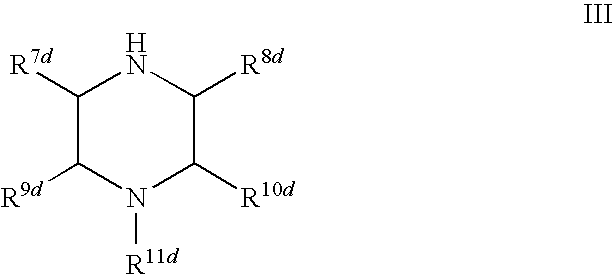
[0000]wherein R7dto R11dhave any of the above given meanings, in a suitable reaction medium, preferably in at least an organic solvent, more preferably in at least an organic solvent selected from the group consisting of tetrahydrofuran or dioxane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium source, even more preferably in the presence of PdCl2(dppf), wherein dppf is 1,1-bis(diphenylphosphino)-ferrocene, and/or at least one auxiliary agent, preferably 1,1-bis(diphenylphosphino)-ferrocene, and/or at least one base, preferably sodium tert-pentoxide, to yield a compound of general formula IV,
[0000]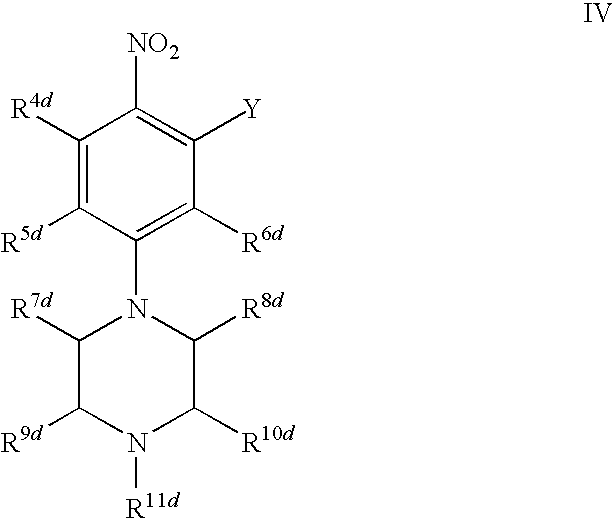
[0000]wherein R4dto R11dhave any of the above given meanings and Ydrepresents a chlorine atom; which is optionally purified and/or isolated, and the compound of general formula IV is reacted with at least one compound of general formula IX,
[0000]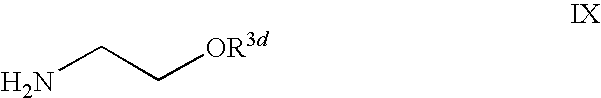
[1173]wherein R3dhas any of the above given meanings, in a suitable reaction medium, preferably in at least an organic solvent, more preferably in at least an organic solvent selected from the group consisting of toluene or dimethoxyethane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium source, even more preferably in the presence of at least a palladium source selected from the group consisting of Pd(OAc)2, wherein OAc is acetate, and Pd2dba3, wherein dba is dibenzylidene acetone, and/or at least one auxiliary agent, preferably (biph)P(tBu)2, wherein biph is biphenyl and tBu is tert-butyl, and/or at least one base, preferably at least one base selected from the group consisting of K3PO4and sodium tert-pentoxide to yield a compound of general formula X,
[0000]
[0000]wherein R3dto R11dhave any of the above given meanings, which is optionally purified and/or isolated.
[1174]Suitable reaction media include organic solvents, such as dialkyl ether, preferably diethyl ether and dimethoxyethane, or a cyclic ether, preferably tetrahydrofuran or dioxane; or a halogenated hydrocarbon, preferably dichloromethane or chloroform; an alcohol, preferably methanol or ethanol; an aprotic solvent, preferably acetonitrile, toluene, pyridine or dimethylformamide, or any other suitable reaction medium. Of course, mixtures of at least two classes of solvents or of at least two solvents of one class may also be used.
[1175]All of above mentioned reactions are preferably carried out in an oven-dried vial. The catalyst, the auxiliary agent, the base and the compound of general formula II, IV, VII or VIII are added in each case and the vial is subsequently evacuated and purged with argon. The organic solvent and the compound of general formula III, V or IX are added and the reaction is carried out in a sealed vial at a temperature between 100° C. and 110° C., preferably at 100° C. in case of tetrahydrofurane or toluene as the organic solvent and at 110° C. in case of dimethoxyethane and dioxane as the organic solvent.
[1176]Suitable reaction conditions for carrying out the reaction between compounds of general formula II, IV, VII or VIII and compounds of general formula III, V or IX are described in the references of J. F. Hartwig et al., J. Am. Chem. Soc. 1996, 118, 7217-7218; S. L. Buchwald et al., J. Am. Chem. Soc. 2002, 124, 6043-6048; S. L. Buchwald et al. J. Am. Chem. Soc. 2002, 124, 7241-7424 and S. L. Buchwald et al., J. Am. Chem. Soc. 2002, 124, 11684-11688. The respective part of the description is hereby incorporated by reference and forms part of the present disclosure.
[1177]The compounds of general formulas IV, VI, VIII and X given above may be purified and/or isolated according to methods well known to those skilled in the art.
[1178]The compounds of general formulas IV, VI, VIII and X may be isolated by evaporating the reaction medium, addition of water and adjusting the pH value to obtain the compound in form of a solid that can be isolated by filtration, or by extraction with a solvent that is not miscible with water such as chloroform and purification by chromatography or recrystallisation from a suitable solvent.
[1179]Preferably, the compounds of general formula IV, VI, VIII and X may be obtained by filtration of the reaction mixture and subsequent separation of the reaction mixture on a TLC plate. Alternatively, the compounds of general formula I may be isolated by addition of water and methanol to the reaction mixture, evaporating the reaction mixture and purifying the residue by preparative HPLC.
[1180]The compounds of general formula II and VII are commercially available or may be prepared according to methods well known in the art, for example, analogous to the methods described in the bibliography of A. McKillop et al., Tetrahedron 1987, 43, 1753. The respective part of the literature description cited above is hereby incorporated by reference and forms part of the disclosure.
[1181]The compounds of general formula III, V and IX are commercially available or may be prepared according to methods well known in the art.
[1182]During some synthetic reactions described above or while preparing the compounds of general formulas III, V, VI, IX or X the protection of sensitive or reactive groups may be necessary and/or desirable. This can be performed by using conventional protective groups like those described in Protective groups in Organic Chemistry, ed. J. F. W. McOmie, Plenum Press, 1973; T. W. Greene & P. G. M. Wuts and Protective Groups in Organic Chemistry, John Wiley & sons, 1991. The respective parts of the description is hereby incorporated by reference and forms part of the disclosure. The protective groups may be eliminated when convenient by means well-known to those skilled in the art.
[1183]If the nitro-substituted phenyl-piperazine compounds of general formula Id are obtained in form of a mixture of stereoisomers, particularly enantiomers or diastereomers, said mixtures may be separated by standard procedures known to those skilled in the art, e.g. chromatographic methods or crystallization with chiral reagents.
[1184]The nitro-substituted phenyl-piperazine compounds of general formula Id and in each case stereoisomers thereof may be obtained in form of a corresponding salt according to methods well known to those skilled in the art, e.g. by reacting said compound with at least one inorganic and/or organic acid, preferably in a suitable reaction medium. Suitable reaction media include, for example, any of the ones given above.
[1185]Preferably as component (A) at least one compound is present which is selected from the group consisting of phenyl-piperazine-derived compounds of general formula (Ie)
[0000]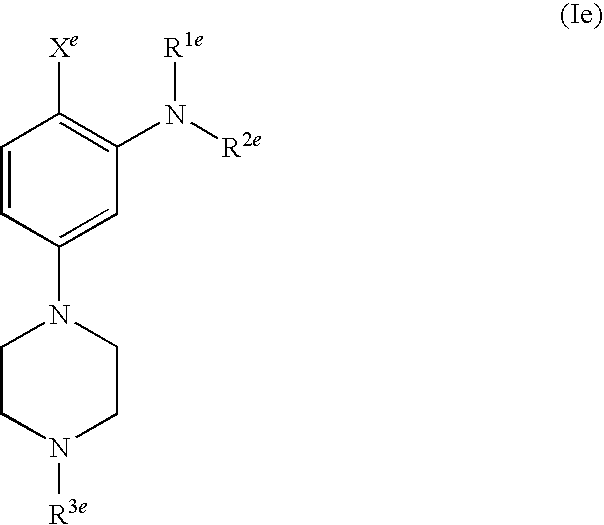
[0000]wherein
Xerepresents —CN, —C(═O)—OH, —C(═O)—OR4e, —O—R5e, —NH2, —NR6e—C(═O)—R7e, —NH—S(═O)2—R8eor —NH—R9e;
R1erepresents a hydrogen atom;
a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group which may contain 1, 2 or 3 heteroatom(s) independently selected from the group consisting of nitrogen, oxygen and sulfur as chain member(s);
R2erepresents a hydrogen atom or a —C(═O)—R10emoiety;
or
R1eand R2etogether with the bridging nitrogen form a nitro (NO2)-group or
an unsubstituted or at least mono-substituted 5- or 6-membered heteroaryl radical which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R3erepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
R4erepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
R5erepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group;
R6erepresents a hydrogen atom or
an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group;
R7erepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
R8erepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group;
R9erepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group;
and
R10erepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1186]Preferred compounds of general formula (Ie) are those, wherein
[0000]Xerepresents —CN, —C(═O)—OH, —C(═O)—OR4e, —O—R5e, —NH2, —NR6e—C(═O)—R7e, —NH—S(═O)2—R8eor —NH—R9e;
R1erepresents
a hydrogen atom; or
an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, furyl (furanyl) and thiophenyl (thiophenyl), whereby said aryl or heteroaryl radical is bonded via a —(CH2)—, —(CH2)—(CH2)—, —(CH2)—(CH2)—(CH2)—, —O—(CH2)—(CH2)—, or —(CH2)—(CH2)—O-group and/or may be unsubstituted or substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, —O—CH3, —O—C2H5, —O—CH2—CH2—CH3, —O—CH(CH3)2, —O—C(CH3)3, F, Cl, Br, —CN, —CF3, —OCF3, —OH and —SH.
R2represents a hydrogen atom or a —C(═O)—R10moiety;
R1eand R2etogether with the bridging nitrogen atom form a nitro group or moiety selected from the group consisting of
[0000]
[0000]whereby each of these aforementioned cyclic moieties may be unsubstituted or substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, F, Cl, Br, I, —CN and —CF3,
R3erepresents a methyl or ethyl radical;
R4erepresents a methyl or ethyl radical;
R5erepresents
an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
or an aryl or heteroaryl radical selected from the group consisting of phenyl, naphthyl, furyl (furanyl) and thiophenyl (thiophenyl), whereby said aryl or heteroaryl radical is bonded via a —(CH2)—, —(CH2)—(CH2)— or —(CH2)—(CH2)—(CH2)— group and/or may be unsubstituted or substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, —O—CH3, —O—C2H5, —O—CH2—CH2—CH3, —O—CH(CH3)2, —O—C(CH3)3, F, Cl, Br, —CN, —CF3, —OCF3, —OH and —SH;
R6erepresents a hydrogen atom, or
a phenyl radical, whereby said phenyl radical may be bonded via a —(CH2)-group and/or may be unsubstituted or substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, —O—CH3, —O—C2H5, F, Cl, Br, —CF3, —OCF3, —OH and —SH;
R7erepresents a methyl or ethyl radical;
R8erepresents
an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl; or
an aryl radical selected from the group consisting of phenyl and naphthyl, whereby said aryl radical may be bonded via a —(CH2)—, or —(CH2)—(CH2)-group and/or may be unsubstituted or substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, —O—CH3, —O—C2H5, —O—CH2—CH2—CH3, —O—CH(CH3)2, —O—C(CH3)3, F, Cl, Br, —CN, —CF3, —OCF3, —OH and —SH,
R9erepresents
an alkyl radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
or an aryl radical selected from the group consisting of phenyl and naphthyl, whereby said aryl radical may be bonded via a —(CH2)—, —(CH2)—(CH2)— or —(CH2)—(CH2)—(CH2)— group and/or may be unsubstituted or substituted with 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, —O—CH3, —O—C2H5, —O—CH2—CH2—CH3, —O—CH(CH3)2, —O—C(CH3)3, F, Cl, Br, —CN, —CF3, —OCF3, —OH and —SH;
R10erepresents a methyl or ethyl radical;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof, respectively.
[1187]Particularly preferred compounds of general formula (Ie) are those selected from the group consisting of
- [1086] 4-(4-Methyl-piperazin-1-yl)-2-phenethylamino-benzoic acid,
- [1087] 2-[(Furan-2-ylmethyl)-amino]-4-(4-methyl-piperazin-1-yl)-benzonitrile,
- [1088] 2-[(Furan-2-ylmethyl)-amino]-4-(4-methyl-piperazin-1-yl)-benzoic acid,
- [1089] 2-Benzylamino-4-(4-methyl-piperazin-1-yl)-benzoic acid methyl ester,
- [1090] 2-Benzylamino-4-(4-methyl-piperazin-1-yl)-benzonitrile,
- [1091] 4-(4-Methyl-piperazin-1-yl)-2-phenethylamino-benzoic acid methyl ester,
- [1092] 2-[(Furan-2-ylmethyl)-amino]-4-(4-methyl-piperazin-1-yl)-benzoic acid methyl ester,
- [1093] 2-Benzylamino-4-(4-methyl-piperazin-1-yl)-benzoic acid,
- [1094] [2-Benzyloxy-5-(4-methyl-piperazin-1-yl)-phenyl]-phenethyl-amine,
- [1095] [2-Benzyloxy-5-(4-methyl-piperazin-1-yl)-phenyl]-furan-2-yl-methyl amine,
- [1096] Benzyl-[2-methoxy-5-(4-methyl-piperazin-1-yl)-phenyl]-amine,
- [1097] [2-Methoxy-5-(4-methyl-piperazin-1-yl)-phenyl]-phenethyl-amine,
- [1098] Furan-2-ylmethyl-[2-methoxy-5-(4-methyl-piperazin-1-yl)-phenyl]-amine,
- [1099] Benzyl-[2-benzyloxy-5-(4-methyl-piperazin-1-yl)-phenyl]-amine,
- [1100] N-[2-Acetyl-(2-phenoxyethyl)-amino]-4-(4-methyl-piperazin-1-yl)-phenyl]-acetamide,
- [1101] N-[4-(4-Methyl-piperazin-1-yl)-2-(2-phenoxy-ethylamino)-phenyl]-acetamide,
- [1102] N-[2-(Acetyl-amino)-4-(4-methyl-piperazin-1-yl)-phenyl]-N-benzyl-acetamide,
- [1103] N-[2-(3,5-Dimethyl-pyrazol-1-yl)-4-(4-methyl-piperazin-1-yl)-phenyl]-acetamide,
- [1104] N-[2-(Acetyl-furan-2-ylmethyl-amino)-4-(4-methyl-piperazin-1-yl)-phenyl]-acetamide,
- [1105] N-[2-Benzylamino-4-(4-methyl-piperazin-1-yl)-phenyl]-acetamide,
- [1106] N-[2-[Furan-2-ylmethyl)-amino]-4-(4-methyl-piperazin-1-yl)-phenyl]-acetamide,
- [1107] N-[2-Amino-5-(4-methyl-piperazin-1-yl)-phenyl]-N-furan-2-ylmethyl-acetamide,
- [1108] N-[2-Amino-4-(4-methyl-piperazin-1-yl)-phenyl]-N-benzyl-acetamide,
- [1109] N-[2-Benzylamino-4-(4-methyl-piperazin-1-yl)-phenyl]-benzenesulfonamide,
- [1110] N-[2-Benzylamino-4-(4-methyl-piperazin-1-yl)-phenyl]-methansulfonamide,
- [1111] 2-Benzyloxy-5-(4-methyl-piperazin-1-yl)-phenylamine,
- [1112] Benzyl-[4-(4-methyl-piperazin-1-yl)-2-nitro-phenyl]-amine and
- [1113] 2-Cyano-(5-piperazin-1-yl-methyl)-2-phenoxy-ethylamine
optionally in form of one of their stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a physiologically acceptable salt thereof, or a corresponding solvate thereof.
[1216]The compounds of general formula Ie are prepared by a process, wherein at least one substituted benzene compound of general formula II,
[0000]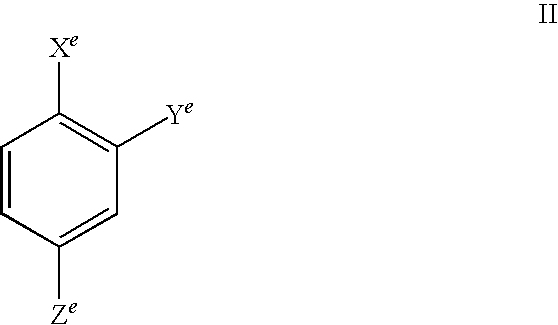
[0000]wherein Xerepresents —CN, —C(═O)—OR4e, —O—R5eor —NO2, R4eand R5ehave any of the above given meanings, Yerepresents a chlorine atom, and Zerepresents a bromine or iodine atom; is reacted with at least one piperazine compound of general formula III,
[0000]
[0000]wherein R3ehas any of the above given meanings, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of tetrahydrofuran, toluene or dioxane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium source, even more preferably in the presence of at least one palladium source selected from the group consisting of Pd(OAc)2, wherein OAc is acetate, and PdCl2(dppf), wherein dppf is 1,1-bis(diphenylphosphino)-ferrocene, and/or at least one auxiliary agent, preferably at least one auxiliary agent selected from the group consisting of 1,1-bis(diphenylphosphino)-ferrocene and 2,2′-bis(diphenylphosphino)-1′1-binaphthyl (BINAP), optionally in form of its enantiomers or a racemate, and/or at least one base, preferably at least one base selected from
the group consisting of sodium tert-pentoxide and Cs2CO3to yield a compound of general formula IV,
[0000]
[0000]wherein Xerepresents —CN, —C(═O)—OR4e, —O—R5eor —NO2, R3e, R4eand R5ehave any of the above given meanings and Yerepresents a chlorine atom; which is optionally purified and/or isolated, and the compound of general formula IV is reacted with at least one compound of general formula V,
[0000]
[0000]wherein R1eand R2ehave any of the above given meanings or one of them represents a protecting group, preferably a —C(═O)—O—C(CH3)3group, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of toluene, dioxane and dimethoxyethane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium source, even more preferably in the presence of at least a palladium source selected from the group consisting of Pd(OAc)2, wherein OAc is acetate, and Pd2dba3, wherein dba is dibenzylidene acetone, and/or at least one auxiliary agent, preferably at least one auxiliary agent selected from the group consisting of (biph)P(tBu)2, wherein biph is biphenyl and tBu is tert-butyl, and 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (xantphos), and/or at least one base, preferably at least one base selected from the group consisting of K3PO4, Cs2CO3and sodium tert-pentoxide to yield a compound of general formula VI,
[0000]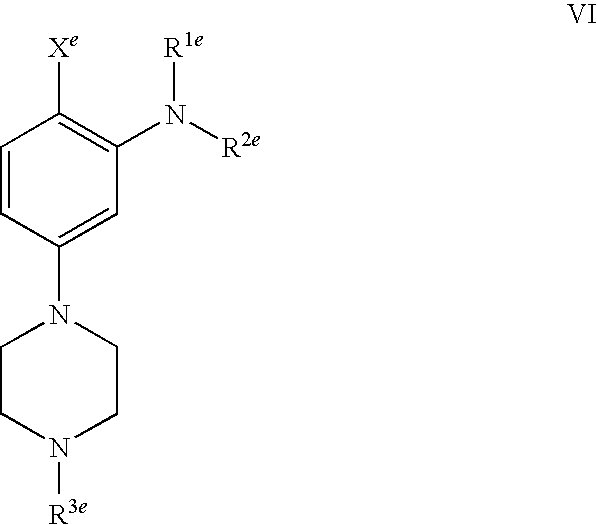
[0000]wherein Xerepresents —CN, —C(═O)—OR4e, —O—R5eor —NO2, R1e, R2ehave any of the above given meanings or one of them represents a protecting group, preferably —C(═O)—O—C(CH3)3and R3e, R4eand R5ehave any of the above given meanings, said compound of general formula VI is being optionally purified and/or isolated,
or at least one substituted benzene compound of general formula IIa,
[0000]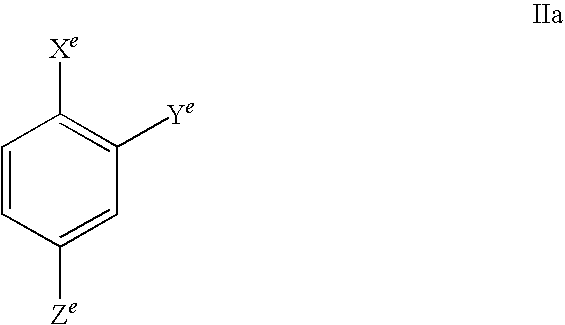
[0000]wherein Xerepresents —CN, —C(═O)—OR4e, —O—R5eor —NO2, R4eand R5ehave any of the above given meanings, Zerepresents a chlorine atom, Yerepresents a bromine or iodine atom, is reacted with at least one compound of general formula V,
[0000]
[0000]wherein R1eand R2ehave any of the above given meanings or one of them represents a protecting group, preferably a —C(═O)—O—C(CH3)3group in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of toluene, dimethoxyethane and dioxane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium and/or copper source, even more preferably in the presence of at least a palladium and/or copper source selected from the group consisting of Pd(OAc)2, wherein OAc is acetate, Pd2dba3, wherein dba is dibenzylidene acetone, and copper(I)iodide, and/or at least one auxiliary agent, preferably at least an auxiliary agent selected from the group consisting of 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (Xantphos), 1,1-bis(diphenylphosphino-ferrocene and P(tBu)3wherein tBu is tert-Butyl, and/or at least one base, preferably at least one base selected from the group consisting of K3PO4, Cs2CO3and trans-1,2-diamino-methylcyclohexane to yield a compound of general formula VII,
[0000]
[0000]wherein Xerepresents —CN, —C(═O)—OR4e, —O—R5eor —NO2, R1eand R2ehave any of the above given meanings or one of them represents a protecting group, preferably a —C(═O)—O—C(CH3)3group, R4eand R5ehave any of the above given meanings, and Y represents a chlorine atom; said compound of general formula being optionally purified and/or isolated, and the compound of general formula VII is reacted with at least one compound of general formula III,
[0000]
[0000]wherein R3ehas any of the above given meanings, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of tetrahydrofuran, toluene or dioxane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium source, even more preferably in the presence of at least one palladium source selected from the group consisting of Pd(OAc)2, wherein OAc is acetate, and PdCl2(dppf), wherein dppf is 1,1-bis(diphenylphosphino)-ferrocene, and/or at least one auxiliary agent, preferably at least one auxiliary agent selected from the group consisting of 1,1-bis(diphenylphosphino)-ferrocene and 2,2′-bis(diphenylphosphino)-1′1-binaphthyl (BINAP), optionally in form of its enantiomers or a racemate, and/or at least one base, preferably at least one base selected from the group consisting of sodium tert-pentoxide and Cs2CO3, to yield a compound of general formula VI,
[0000]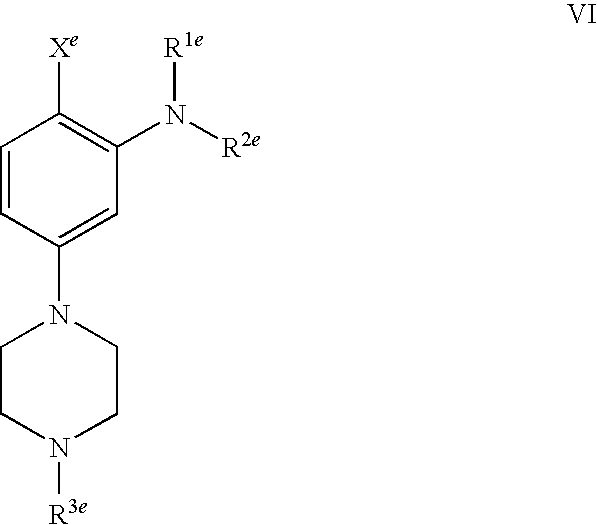
[0000]wherein Xerepresents —CN, —C(═O)—OR4e, —O—R5eor —NO2, R1eand R2ehave any of the above given meanings or one of them represents a protecting group, preferably a —C(═O)—O—C(CH3)3group, and R3e, R4eand R5ehave any of the above given meanings, and said compound of general formula VI is optionally purified and/or isolated,
or
at least one substituted benzene compound of general formula VIII,
[0000]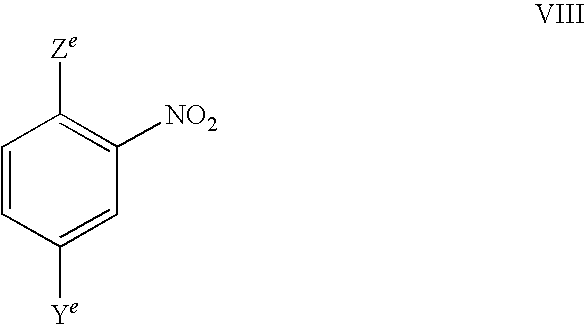
[0000]wherein Zerepresents bromine or iodine and Yerepresents chlorine, is reacted with at least one compound of general formula IX,
[0000]
[0000]wherein R6ehas any of the above given meanings and PG represents a protecting group, preferably a —C(═O)—O—C(CH3)3group, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of toluene and dioxane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium and/or copper source, even more preferably in the presence of at least a palladium and/or copper source selected from the group consisting of Pd(OAc)2wherein OAc is acetate, Pd2dba3wherein dba is dibenzylidene acetone and copper(I)iodide, and/or at least one auxiliary agent, preferably at least an auxiliary agent selected from the group consisting of 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (Xantphos), 1,1-bis(diphenylphosphino-ferrocene and P(tBu)3wherein tBu is tert-Butyl, and/or at least one base, preferably at least one base selected from the group consisting of K3PO4, Cs2CO3and trans-1,2-diamino-methylcyclohexane to yield a compound of general formula XI,
[0000]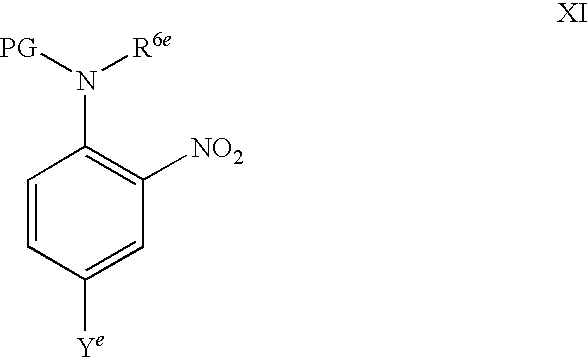
[0000]wherein R6ehas any of the above given meanings, PG represents a protecting group, preferably a —C(═O)—O—C(CH3)3group and Yerepresents chlorine; which is optionally purified and/or isolated, and the compound of general formula XI reacted with at least one compound of general formula III,
[0000]
[0000]wherein R3ehas any of the above given meanings, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of tetrahydrofuran, toluene or dioxane, preferably in the presence of at least one catalyst, more preferably in the presence of at least a palladium source, even more preferably in the presence of at least one palladium source selected from the group consisting of Pd(OAc)2, wherein OAc is acetate, and PdCl2(dppf), wherein dppf is 1,1-bis(diphenylphosphino)-ferrocene, and/or at least one auxiliary agent, preferably at least one auxiliary agent selected from the group consisting of 1,1-bis(diphenylphosphino)-ferrocene and 2,2′-bis(diphenylphosphino)-1′1-binaphthyl (BINAP), optionally in form of its enantiomers or a racemate, and/or at least one base, preferably at least one base selected from the group consisting of sodium tert-pentoxide and Cs2CO3, to yield a compound of general formula XII,
[0000]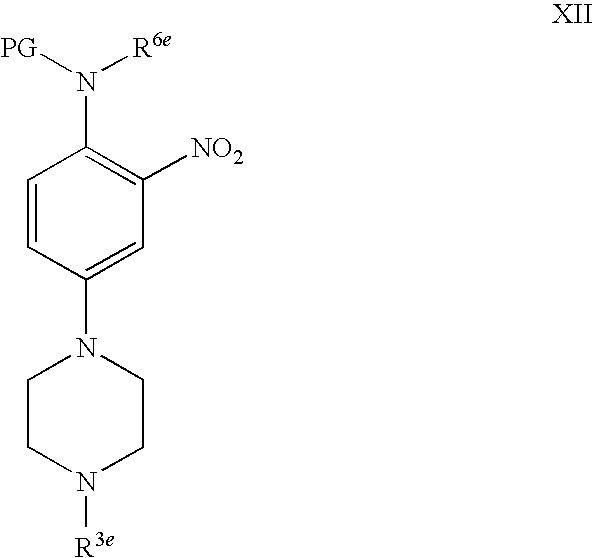
[0000]wherein R3eand R6ehave any of the above given meanings and PG represents a protecting group, preferably a —C(═O)—O—C(CH3)3group, which is optionally purified and/or isolated, and the compound of general formula XII is reacted with at least one acid in a suitable reaction medium to yield a compound of general formula XIII,
[0000]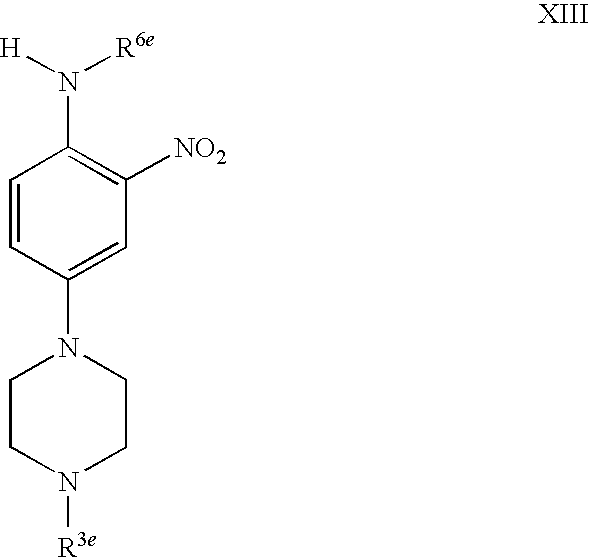
[0000]wherein R3eand R6ehave any of the above given meanings, which is optionally purified and/or isolated, and the compound of general formula is reacted with hydrogen in the presence of at least one catalyst, preferably in the presence of at least one palladium source, more preferably in the presence of palladium on charcoal, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in an organic solvent selected from the group consisting of dioxane, tetrahydrofuran and diethyl ether, to yield a compound of general formula XIV,
[0000]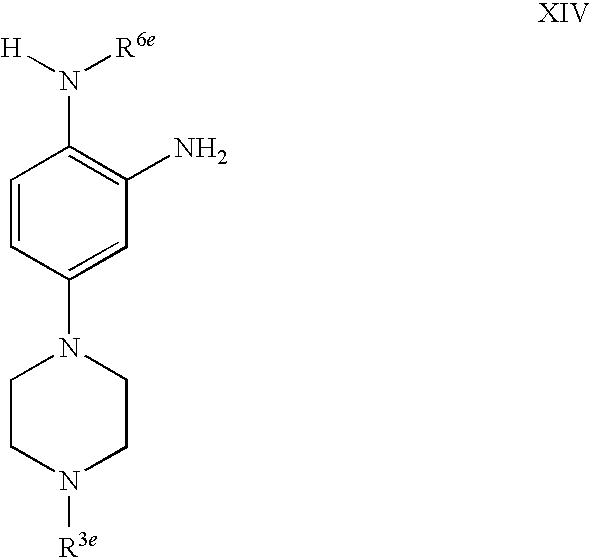
[1217]wherein R3eand R6ehave any of the above given meanings, which is optionally purified and/or isolated, and the compound of general formula XIV is reacted with at least one compound of general formula R7e—C(═O)—O—C(═O)—R7e, wherein R7eany of the above given meanings, and/or at least one compound of general formula R10e—C(═O)—O—C(═O)—R10e, wherein R10ehas any of the above given meanings, optionally in the presence of at least one base, preferably in the presence of at least one organic base, more preferably in the presence of at least an organic base selected from the group consisting of pyridine, triethylamine and diisopropylethylamine, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of dioxane, tetrahydrofuran and diethyl ether, to yield a compound of general formula I, wherein Xerepresents —NR6e— C(═O)R7e, R1erepresents a hydrogen atom, R2erepresents a hydrogen atom or a —C(═O)—R10e-moiety and R3e, R6e, R7eand R10ehave any of the above given meanings, which is optionally purified and/or isolated,
[0000]and/or at least one compound of general formula VI, wherein Xerepresents —CN, —C(═O)—OR4eor —O—R5e, R1eand R2ehave any of the above given meanings or one of them represents a protecting group, preferably a —C(═O)—O—C(CH3)3-group, R3e, R4eand R5ehave any of the above given meanings, is reacted with at least one acid, preferably at least one acid selected from the group consisting of sulfuric acid, hydrochloric acid and acetic acid, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of dioxane and tetrahydrofuran, to yield a compound of general formula I, wherein Xerepresents —CN, —C(═O)—OR4eor —O—R5e, R1eand R3eto R5ehave any of the above given meanings and R2erepresents hydrogen, which is optionally purified and/or isolated,
and optionally at least one compound of general formula I, wherein Xerepresents —CN, —C(═O)—OR4eor —O—R5e, R1eand R3eto R5ehave any of the above given meanings and R2erepresents hydrogen, is reacted with hydrogen in the presence of at least one catalyst, preferably in the presence of at least one palladium source, more preferably in the presence of palladium on charcoal, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in an organic solvent selected from the group consisting of dioxane, tetrahydrofuran and diethyl ether, to yield a compound of general formula I, wherein Xerepresents —CN, —C(═O)—OR4eor —O—R5e, R3eto R5ehave any of the above given meanings and R1eand R2eeach represent hydrogen,
and/or
at least one compound of general formula VI, wherein Xerepresents —C(═O)—OR4e, R1eand R2ehave any of the above given meanings or one of them represents a protecting group, preferably a —C(═O)—O—C(CH3)3-group, R3eand R4ehave any of the above given meanings, is reacted with at least one base, preferably at least one metal hydroxide, more preferably at least one metal hydroxide selected from the group consisting of lithium hydroxide and potassium hydroxide, in a suitable reaction medium, preferably in a mixture of at least one organic solvent and water, more preferably in a mixture of at least one organic solvent selected from the group consisting of dioxane, ethanol and methanol and water, to yield a compound of general formula XV, wherein Xerepresents —C(═O)—OH, R1eand R2ehave any of the above given meanings or one of them represents a protecting group, preferably a —C(═O)—O—C(CH3)3-group, R3ehas any of the above given meanings, which is optionally purified and/or isolated and at least one compound of general formula XV is reacted with at least one acid, preferably at least one acid selected from the group consisting of sulfuric acid, hydrochloric acid and acetic acid, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of dioxane and tetrahydrofuran, to yield a compound of general formula I, wherein Xerepresents —C(═O)—OH, R1eand R3ehave any of the above given meanings and R2erepresents hydrogen, which is optionally purified and/or isolated,
and/or
at least one compound of general formula VI, wherein Xerepresents —NO2, R1eand R2ehave any of the above given meanings or one of them represents a protecting group, preferably a —C(═O)—O—C(CH3)3-group and R3ehas any of the above given meanings, is reacted with hydrogen in the presence of at least one catalyst, preferably in the presence of at least one palladium source, more preferably in the presence of palladium on charcoal, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in an organic solvent selected from the group consisting of dioxane, tetrahydrofuran and diethyl ether, to yield a compound of general formula XVI, wherein Xerepresents —NH2, R1eand R2ehave any of the above given meanings or one of them represents a protecting group, preferably a —C(═O)—O—C(CH3)3-group, and R3ehas any of the above given meanings, which is optionally purified and/or isolated, and at least one compound of general formula XVI, is reacted with at least one acid, preferably at least one acid selected from the group consisting of sulfuric acid, hydrochloric acid and acetic acid, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of dioxane and tetrahydrofuran, to yield a compound of general formula I, wherein Xerepresents —NH2, R1eand R3ehave any of the above given meanings and R2erepresents hydrogen, which is optionally purified and/or isolated,
and optionally at least one compound of general formula I, wherein Xerepresents —NH2, R1eand R3ehave any of the above given meanings and R2erepresents hydrogen, is reacted with at least one compound of general formula R7e—C(═O)—O—C(═O)—R7eand/or at least one compound of general formula R10e—C(═O)—O—C(═O)—R10e, wherein R7eand R10ehave any of the above given meanings optionally in the presence of at least one base, preferably in the presence of at least one organic base, more preferably in the presence of at least an organic base selected from the group consisting of pyridine, triethylamine and diisopropylethylamine, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of dioxane, tetrahydrofuran and diethyl ether, to yield a compound of general formula I, wherein Xerepresents —NH—C(═O)—R7eand R1eto R3ehave any of the above given meanings, which is optionally purified and/or isolated,
and/or optionally at least one compound of general formula I, wherein Xerepresents —NH2and R1eand R3ehave any of the above given meanings and R2erepresents hydrogen, is reacted with at least one compound of general formula R8e—S(═O)—W, wherein R8ehas any of the above given meanings and W represents a halogen atom, preferably a chlorine atom, optionally in the presence of at least one base, preferably in the presence of at least one organic base, more preferably in the presence of an organic base selected from the group consisting of pyridine, triethylamine and diisopropylethylamine, in a suitable reaction medium, preferably in at least one organic solvent, more preferably in at least one organic solvent selected from the group consisting of dioxane, tetrahydrofuran and diethyl ether, to yield a compound of general formula I, wherein Xerepresents —NH—S(═O)2—R8eand R1e, R3eand R8ehave any of the above given meanings and R2erepresents hydrogen, which is optionally purified and/or isolated.
[1218]Suitable reaction media include organic solvents, such as dialkyl ether, preferably diethyl ether and dimethoxyethane, or a cyclic ether, preferably tetrahydrofuran or dioxane; or a halogenated hydrocarbon, preferably dichloromethane or chloroform; an alcohol, preferably methanol or ethanol; an aprotic solvent, preferably acetonitrile, pyridine, toluene or dimethylformamide, or any other suitable reaction medium. Of course, mixtures of at least two classes of solvents or of at least two solvents of one class may also be used.
[1219]If the above mentioned reactions are carried out in an oven-dried vial, the catalyst, the auxiliary agent, the base and the compound of general formula II, IIa, IV, VII, VIII or XI are added in each case and the vial is subsequently evacuated and purged with argon. The organic solvent and the compound of general formula III, V and IX are added and the reaction is carried out in a sealed vial at a temperature between 100° C. and 110° C., preferably at 100° C. in case of tetrahydrofurane or toluene as the organic solvent and at 110° C. in case of dimethoxyethane and dioxane as the organic solvent.
[1220]Suitable reaction conditions for carrying out the reaction between compounds of general formula II, IIa, IV, VII, VIII or XI and compounds of general formula III, V and IX are described in the references of J. F. Hartwig et al., J. Am. Chem. Soc. 1996, 118, 7217-7218; S. L. Buchwald et al., J. Org. Chem. 2000, 65, 1144-1157;
[1221]S. L. Buchwald et al., J. Am. Chem. Soc. 2002, 124, 6043-6048; S. L. Buchwald et al. J. Am. Chem. Soc. 2002, 124, 7241-7424 and S. L. Buchwald et al., J. Am. Chem. Soc. 2002, 124, 11684-11688. The respective part of the description is hereby incorporated by reference and forms part of the present disclosure.
[1222]The compounds of general formula I, IV, VI, VII, XI, XII, XIII, XIV, XV and XVI may be isolated by evaporating the reaction medium, addition of water and adjusting the pH value to obtain the compound in form of a solid that can be isolated by filtration, or by extraction with a solvent that is not miscible with water such as chloroform and purification by chromatography or recrystallisation from a suitable solvent.
[1223]Preferably, the compounds of general formula I, IV, VI, VII, XI, XII, XIII, XIV, XV and XVI may be obtained by filtration of the reaction mixture and subsequent separation of the reaction mixture on a TLC plate. Alternatively, the compounds of general formula I, IV, VI, VII, XI, XII, XIII, XIV, XV and XVI may be isolated by addition of water and methanol to the reaction mixture, evaporating the reaction mixture and purifying the residue by preparative HPLC.
[1224]The compounds of general formula II, IIa, VIII and IX are commercially available or may be prepared according to methods well known in the art, for example, analogous to the methods described in the bibliography of A. McKillop et al., Tetrahedron 1987, 43, 1753. The respective part of the literature description cited above is hereby incorporated by reference and forms part of the disclosure.
[1225]During some synthetic reactions described above or while preparing the compounds of general formulas II, IIa, VIII and IX the protection of sensitive or reactive groups may be necessary and/or desirable. This can be performed by using conventional protective groups like those described in Protective groups in Organic Chemistry, ed. J. F. W. McOmie, Plenum Press, 1973; T. W. Greene & P. G. M. Wuts and Protective Groups in Organic Chemistry, John Wiley & sons, 1991. The respective parts of the description are hereby incorporated by reference and forms part of the disclosure. The protective groups may be eliminated when convenient by means well-known to those skilled in the art.
[1226]If the substituted phenyl-piperazine compounds of general formula Ie are obtained in form of a mixture of stereoisomers, particularly enantiomers or diastereomers, said mixtures may be separated by standard procedures known to those skilled in the art, e.g. chromatographic methods or crystallization with chiral reagents.
[1227]The substituted phenyl-piperazine compounds of general formula Ie and in each case stereoisomers thereof may be obtained in form of a corresponding salt according to methods well known to those skilled in the art, e.g. by reacting said compound with at least one inorganic and/or organic acid, preferably in a suitable reaction medium. Suitable reaction media include, for example, any of the ones given above.
[1228]In another embodiment of the present invention as component (A) at least one compound is present which is selected from the group consisting of tetrahydroisoquinoline-derived sulfonamide compounds of general formula (If)
[0000]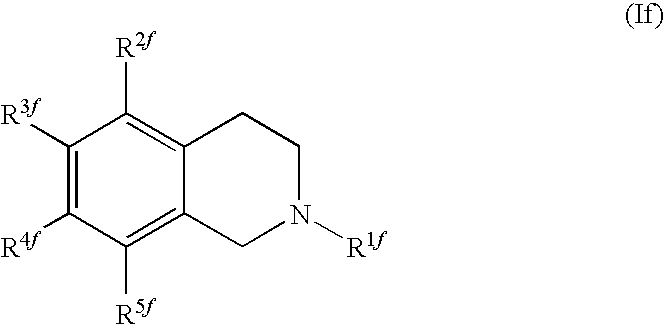
[0000]wherein
R1frepresents a hydrogen atom; a —C(═O)—OR37fmoiety;
a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
R2f, R3f, R4fand R5f, independently of one another, each represent a hydrogen atom; F, Cl, Br, I, —NO2; —NH2; —SH; —OH; —CN; —C(═O)—OH; —C(═O)—H; —S(═O)2—OH; —C(═O)—NH2; —S(═O)2—NH2; —C(═O)—R6f; —S(═O)—R7f; —S(═O)2—R7f; —OR8f; —SR9f; —C(═O)—OR10f; —N(R11f)—S(═O)2—R12f; —NR13fR14f; —NH—R15f; —C(═O)—NR16fR17f; C(═O)—NHR18f;
a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group and which may be condensed with an unsubstituted or at least mono-substituted saturated, unsaturated or aromatic mono- or bicyclic ring system;
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group and which may be condensed with an unsubstituted or at least mono-substituted saturated or unsaturated, but not aromatic, mono- or bicyclic ring system;
with the proviso that at least one of the substituents R2f, R3f, R4fand R5frepresents a —N(R11f)—S(═O)2—R12fmoiety;
R6f, R7f, R8f, R9f, R10f, R13f, R14f, R15f, R16f, R17fand R18f,
independently of one another, each represent a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group and which may be condensed with an unsubstituted or at least mono-substituted saturated, unsaturated or aromatic mono- or bicyclic ring system;
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group and which may be condensed with an unsubstituted or at least mono-substituted saturated or unsaturated, but not aromatic, mono- or bicyclic ring system;
R11frepresents a hydrogen atom or a linear or branched, saturated or
unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
R12frepresents a phenyl radical of general formula (Af),
[0000]
[0000]wherein
R19f, R20f, R21f, R22fand R38f, independently of one another, each represent a hydrogen atom; F, Cl, Br, I, —NO2; —NH2; —SH; —OH; —CN; —C(═O)—OH; —C(═O)—H; —S(═O)2—OH; —C(═O)—NH2; —S(═O)2—NH2; —C(═O)—R23f; —S(═O)—R24f; —S(═O)2—R24f; —OR25f; —SR26f; —C(═O)—OR27f; —N(R28f)—S(═O)2—R29f; —NH—S(═O)2—R30f; —NR31fR32f; —NH—R33f; —C(═O)—NHR34f; —C(═O)—NR35fR36f; a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical; or a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group and/or which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system;
an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system and/or which may be bonded via a linear or branched, unsubstituted or at least mono-substituted alkylene, alkenylene or alkinylene group;
or a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be condensed with an unsubstituted or at least mono-substituted saturated, unsaturated or aromatic mono- or bicyclic ring system;
R23f, R27f, R28f, R29fand R30f, independently of one another, each represent
a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group and which may be condensed with an unsubstituted or at least mono-substituted saturated, unsaturated or aromatic mono- or bicyclic ring system;
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group and which may be condensed with an unsubstituted or at least mono-substituted saturated or unsaturated, but not aromatic, mono- or bicyclic ring system;
R24f, R26f, R31f, R32fand R33f, each represent a linear or branched,
saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group and which may be condensed with an unsubstituted or at least mono-substituted saturated, unsaturated or aromatic mono- or bicyclic ring system;
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be condensed with an unsubstituted or at least mono-substituted saturated or unsaturated, but not aromatic, mono- or bicyclic ring system;
R25f, R34f, R35fand R36f, represents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
and R37frepresents a linear or branched, saturated or unsaturated, unsubstituted or at least mono-substituted aliphatic radical;
a saturated or unsaturated, unsubstituted or at least mono-substituted, optionally at least one heteroatom as a ring member containing cycloaliphatic radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group and which may be condensed with an unsubstituted or at least mono-substituted saturated, unsaturated or aromatic mono- or bicyclic ring system;
or an unsubstituted or at least mono-substituted aryl or heteroaryl radical, which may be bonded via a linear or branched alkylene, alkenylene or alkinylene group and which may be condensed with an unsubstituted or at least mono-substituted saturated or unsaturated, but not aromatic, mono- or bicyclic ring system;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a salt thereof, or a corresponding solvate thereof.
[1229]Preferred compounds of general formula (If) are those, wherein
[0000]R1frepresents a hydrogen atom; a —C(═O)—OR37fmoiety; a radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, n-pentyl, n-hexyl, —CH2—NH2, —CH2—NH—CH3, —CH2—N(CH3)2, —CH2—N(C2H5)2, —CH2—NH—C2H5, —CH2—CH2—NH2, —CH2—CH2—NH—CH3, —CH2—CH2—N(CH3)2, —CH2—CH2—N(C2H5)2, —CH2—CH2—NH—C2H5, —CH2—CH2—CH2—NH—CH3, —CH2—CH2—CH2—N(CH3)2, —CH2—CH2—CH2—N(C2H5)2and —CH2—CH2—CH2—NH—C2H5; or a (hetero)cycloaliphatic radical selected from the group consisting of imidazolidinyl, aziridinyl, azetidinyl, pyrrolidinyl, piperidinyl, morpholinyl, thiomorpholinyl, piperazinyl, pyrazolidinyl and azepanyl, which may be bonded via a —(CH2)1, 2 or 3— group and which may be unsubstituted or optionally substituted with 1, 2, 3, 4 or 5 substituents) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl and isobutyl;
R2f, R3f, R4fand R5f, independently of one another, each represent a hydrogen atom;
F, Cl, Br, I, —NO2; —O—CH3; —O—C2H5; —O—CF3; —O—CFH2; —O—CF2H; —O—CH2—CF3; —O—CF2—CF3; —S—CH3; —S—C2H5; —S—CF3; —S—CFH2; —S—CF2H; —S—CH2—CF3; —S—CF2—CF3; —N(R11f)—S(═O)2—R12f; or a radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, tert-butyl, —CF3, —CFH2, —CF2H, —CH2—CF3and —CF2—CF3;
with the proviso that at least one of the substituents R2f, R3f, R4fand R5frepresents a —N(R11f)—S(═O)2—R12fmoiety;
R11frepresents a hydrogen atom, —S(═O)2—R12for an alkyl radical selected from
the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl and tert-butyl;
R12frepresents a phenyl radical of general formula (Af),
[0000]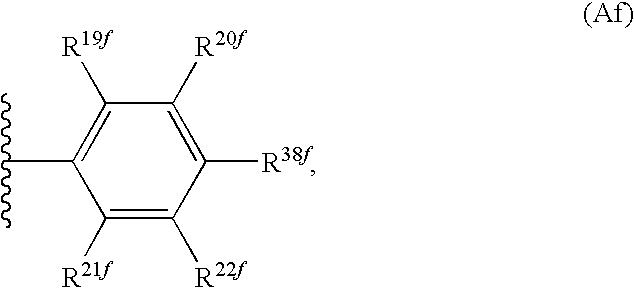
[0000]wherein
R19f, R20f, R21f, R22fand R38f, independently of one another, each represent a hydrogen atom; F, Cl, Br, I, —NO2; —NH2; —SH; —OH; —CN; —C(═O)—OH; —C(═O)—H; —C(═O)—CH3; —C(═O)—C2H5; —O—CH3; —O—C2H5; —O—CF3; —O—CFH2; —O—CF2H; —O—CH2—CF3; —O—CF2—CF3; —S—CH3; —S—C2H5; —S—CF3; —S—CFH2; —S—CF2H; —S—CH2—CF3; —S—CF2—CF3; —C(═O)—OCH3; —C(═O)—OC2H5; methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, —CF3, —CF2H, —CFH2, —CH2—CF3and —CF2—CF3;
a radical selected from the group consisting of naphthyl, [1,3]-benzodioxolyl, [1,4]-benzodioxanyl, benzo[b]furanyl, benzo[b]thiophenyl, benzo[2,1,3]thiadiazolyl, [1,2,3]-benzothiadiazolyl, [2,1,3]-benzoxadiazolyl, [1,2,3]-benzoxadiazolyl, benzoxazolyl, benzothiazolyl, benzisoxazolyl, benzisothiazolyl and imidazo[2,1-b]thiazolyl, which may be unsubstituted or optionally substituted with 1, 2, 3, 4 or 5 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, n-pentyl, —O—CH3, —O—C2H5, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —CF2H and —CFH2;
or a radical selected from the group consisting of pyridinyl, pyrrolyl, imidazolyl, pyrazolyl, triazolyl, pyridazinyl, pyrimidinyl and pyrazinyl, which may be unsubstituted or optionally substituted with 1, 2, 3, 4 or 5 substituent(s) independently selected from the group consisting of F, Cl, Br, I, —NO2; methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, —CF3, —CF2H, —CFH2, —CH2—CF3and —CF2—CF3;
and R37frepresents a radical selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, n-pentyl, n-hexyl, fluorenyl, fluorenylmethyl, phenyl, benzyl and naphthyl;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a salt thereof, or a corresponding solvate thereof.
[1230]Particularly preferred compounds of general formula (If) are those selected from the group consisting of
- [1114] N-(1,2,3,4-tetrahydroisoquinolin-6-yl)naphthalene-1-sulfonamide hydrochloride,
- [1115] 2,2-dimethyl-6-(N-methylnaphthalene-1-sulfonamido)-1,2,3,4-tetrahydroisoquinolinium iodide,
- [1116] N-(2-methyl-1,2,3,4-tetrahydroisoquinolin-6-yl)naphthalene-1-sulfonamide hydrochloride,
- [1117] 5-chloro-3-methyl-N-(1,2,3,4-tetrahydroisoquinolin-6-yl)benzo[b]thiophene-2-sulfonamide hydrochloride,
- [1118] 5-chloro-3-methyl-N-(2-methyl-1,2,3,4-tetrahydroisoquinolin-6-yl)benzo[b]thiophene-2-sulfonamide hydrochloride,
- [1119] 4-methyl-N-(1,2,3,4-tetrahydroisoquinolin-6-yl)naphthalene-1-sulfonamide hydrochloride,
- [1120] 4-methyl-N-(2-methyl-1,2,3,4-tetrahydroisoquinolin-6-yl)naphthalene-1-sulfonamide hydrochloride,
- [1121] N-(1,2,3,4-tetrahydroisoquinolin-6-yl)naphthalene-2-sulfonamide hydrochloride,
- [1122] N-(2-methyl-1,2,3,4-tetrahydroisoquinolin-6-yl)naphthalene-2-sulfonamide hydrochloride,
- [1123] 6-chloro-N-(1,2,3,4-tetrahydroisoquinolin-6-yl)imidazo[2,1-b]thiazole-5-sulfonamide hydrochloride,
- [1124] 2-methoxy-5-methyl-N-(1,2,3,4-tetrahydroisoquinolin-6-yl)benzenesulfonamide hydrochloride,
- [1124] N-(1,2,3,4-tetrahydroisoquinolin-6-yl)pyridine-3-sulfonamide dihydrochloride,
- [1126] 6-(naphthalene-1-sulfonylamino)-3,4-dihydro-1H-isoquinoline-2-carboxylic acid tert-butyl ester,
- [1127] 6-(5-chloro-3-methyl-benzo[b]thiophene-2-sulfonylamino)-3,4-dihydro-1H-isoquinoline-2-carboxylic acid tert-butyl ester,
- [1128] 6-(4-methyl-naphthalene-1-sulfonylamino)-3,4-dihydro-1H-isoquinoline-2-carboxylic acid tert-butyl ester,
- [1129] 6-(naphthalene-2-sulfonylamino)-3,4-dihydro-1H-isoquinoline-2-carboxylic acid tert-butyl ester,
- [1130] 6-(2-methoxy-5-methyl-benzenesulfonylamino)-3,4-dihydro-1H-isoquinoline-2-carboxylic acid tert-butyl ester and
- [1131] 6-(pyridine-3-sulfonylamino)-3,4-dihydro-1H-isoquinoline-2carboxylic acid tert-butyl ester;
optionally in form of one of its stereoisomers, preferably enantiomers or diasteromers, a racemate or in form of a mixture of at least two of its stereoisomers, preferably enantiomers and/or diastereomers, in any mixing ratio, or a salt thereof, or a corresponding solvate thereof.
[1249]The compounds of general formula If are prepared by a process, wherein at least one compound of general formula IV,
[0000]
[0000]wherein R12fhas the meaning given above and X represents a leaving group, preferably a halogen atom, particularly preferably a chlorine atom, is reacted with at least one compound of general formula V,
[0000]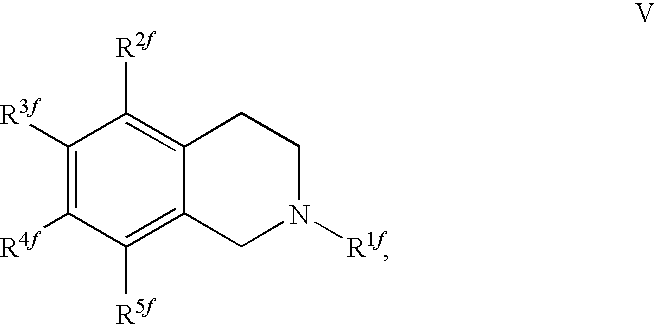
[0000]wherein R1fto R5fhave the meaning given above, with the proviso that at least one substituent of the group consisting of R2f, R3f, R4fand R5frepresents a —N(H)(R11f) moiety, wherein R11fhas the meaning given above, or a protected derivative thereof, in a reaction medium, preferably in a reaction medium selected from the group consisting of pyridine, chloroform, dichloromethane, tetrahydrofurane and mixtures thereof, preferably in the presence of at least one base, more preferably in the presence of at least one base selected from the group consisting of triethylamine, diisopropylethylamine and diethylisopropylamine, preferably at a temperature between 0° C. and 30° C.
[1250]If the substituted tetrahydroisoquinoline compounds of general formula If are obtained in form of a mixture of stereoisomers, particularly enantiomers or diastereomers, said mixtures may be separated by standard procedures known to those skilled in the art, e.g. chromatographic methods or crystallization with chiral reagents.
[1251]The substituted tetrahydroisoquinoline compounds of general formula If and in each case stereoisomers thereof may be obtained in form of a corresponding salt according to methods well known to those skilled in the art, e.g. by reacting said compound with at least one inorganic and/or organic acid, preferably in a suitable reaction medium. Suitable reaction media include, for example, any of the ones given above.
[1252]Compounds of general formula IV are in most cases commercially available or may be prepared by processes known to those skilled in the art.
[1253]Compounds of general formula V are in most cases commercially available or may be prepared by processes known to those skilled in the art.
[1254]In particular, 1,2,3,4-tetrahydroisoquinoline compounds with an amino group in position 5 can be prepared starting from 5-nitro-1,2,3,4-tetrahydroisoquinoline compounds. A process for the preparation of the latter compounds is described in K. V. Rao et al., Journal of Heterocyclic Chemistry, 1973, 10, 213 to 215.
[1255]In particular, 1,2,3,4-tetrahydroisoquinoline compounds with an amino group in position 6 are commercially available or can be prepared starting from 6-nitro-1,2,3,4-tetrahydroisoquinoline compounds. A process for the preparation of the latter compounds is described in G. J. Quallich, Journal of Organic Chemistry, 1998, 63, 4116 to 4119.
[1256]1,2,3,4-tetrahydroisoquinoline compounds with a nitro group in position 6 or 8 may be prepared by established procedures described in M. Tercel, Journal of Medicinal Chemistry, 1996, 39, 1084 to 1094.
[1257]In particular, 1,2,3,4-tetrahydroisoquinoline compounds with an amino group in position 7 are commercially available or can be prepared starting from 7-nitro-1,2,3,4-tetrahydroisoquinoline compounds. A process for the preparation of the latter compounds is described in J. F. Ajao et al., Journal of Heterocyclic Chemistry, 1985, 22, 329 to 331.
[1258]The N-methyl-8-amino-substituted 1,2,3,4-tetrahydroisoquinoline compounds were prepared by bromination and nitration of the corresponding 1,2,3,4-tetrahydroisoquinolines followed by two-step standard reduction conditions as described in M. Rey, Helvetica Chimica Acta, 1985, 66, 1828 to 1834.
[1259]If any of the substituents in any of the above defined formulae represents or comprises a (hetero)cycloaliphatic radical, preferably a C3-9cycloalkyl radical or a C4-9cycloalkenyl radical, or a heterocyclic ring, preferably a 3- to 8-membered heterocyclic ring, said (hetero)cycloaliphatic radical, heterocyclic ring, C3-9cycloalkyl radical or C4-9cycloalkenyl radical may—if not defined otherwise—be unsubstituted or substituted by one or more substituents, preferably unsubstituted or optionally substituted with 1, 2, 3, 4 or 5 substituent(s). Said substituent(s) may preferably be selected independently from the group consisting of oxo (═O), thioxo (═S), C1-5-alkyl, —O—Cl—5-alkyl, —S—Cl—5-alkyl, —C(═O)—OH, —C(═O)—C1-5-alkyl, —C(═O)—O—C1-5-alkyl, —O—C(═O)—C1-5-alkyl, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2, —NH(C1-5-alkyl), —N(C1-5-alkyl)2, —NO2, —CHO, —CF2H, —CFH2, —C(═O)—NH2, —C(═O)—NH(C1-5-alkyl), —C(═O)—N(C1-5-alkyl)2, —S(═O)2—C1-5-alkyl, —S(═O)2-phenyl, phenyl, phenoxy and benzyl; whereby in each occurrence C1-5-alkyl may be linear or branched and whereby said cyclic substituents may be unsubstituted or substituted by 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, methoxy, ethoxy, F, Cl, Br, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2and —NO2.
[1260]More preferably said substituents may be selected independently from the group consisting of oxo (═O), thioxo (═S), methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, n-pentyl, —O—CH3, —O—C2H5, —O—CH2—CH2—CH3, —O—CH(CH3)2, —O—C(CH3)3, —S—CH3, —S—C2H5, —S—CH2—CH2—CH3, —S—CH(CH3)2, —S—C(CH3)3, —C(═O)—OH, —C(═O)—O—CH3, —C(═O)—O—C2H5, —C(═O)—O—CH3—CH3—CH3, —C(═O)—O—CH(CH3)2, —C(═O)—O—C(CH3)3, —C(═O)—CH3, —C(═O)—C2H5, —C(═O)—CH3—CH3—CH3, —C(═O)—CH(CH3)2, —C(═O)—C(CH3)3, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2, —NH—CH3, —NH—C2H5, —NH—CH2—CH2—CH3, —NH—CH(CH3)2, —NH—C(CH3)3, —N(CH3)2, —N(C2H5)2, —NO2, —CHO, —CF2H, —CFH2, —C(═O)—NH2, —C(═O)—NH—CH3, —C(═O)—NH—C2H5, —C(═O)—N(CH3)2, —C(═O)—N(C2H5)2, —S(═O)2—CH3, —S(═O)2-phenyl, phenyl, phenoxy and benzyl; whereby in each occurrence said cyclic substituents may be unsubstituted or substituted by 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, methoxy, ethoxy, F, Cl, Br, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2and —NO2.
[1261]If any of the substituents in any of the above defined formulae represents or comprises a cycloaliphatic radical, C3-9cycloalkyl radical or C4-9cycloalkenyl radical which contains one or more, preferably 1, 2 or 3 heteroatom(s) as ring member(s), unless defined otherwise, each of these heteroatom(s) may preferably be selected independently from the group consisting of N, O and S.
[1262]If any of the substituents in any of the above defined formulae represents or comprises a heterocyclic ring which contains at least one further, preferably 1 or 2 further heteroatom(s) as ring member(s), unless defined otherwise, each of these heteroatom(s) may preferably be selected independently from the group consisting of N, O and S.
[1263]Suitable saturated or unsaturated, optionally at least one heteroatom as ring member containing cycloaliphatic radicals, C3-9cycloalkyl radicals or C4-9cycloalkenyl radicals may preferably be selected from the group consisting of cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, cyclononyl, cyclopentenyl, cyclohexenyl, cycloheptenyl, cyclooctenyl, pyrrolidinyl, piperidinyl, piperazinyl, homopiperazinyl, morpholinyl, aziridinyl, azetidinyl, imidazolidinyl, thiomorpholinyl, pyrazolidinyl, tetrahydrofuranyl, tetrahydrothiophenyl, azepanyl, diazepanyl, azocanyl, (2,5)-dihydrofuranyl, (2,5)-dihydrothiophenyl, (2,3)-dihydrofuranyl, (2,3)-dihydrofuranyl, (2,5)-dihydro-1H-pyrrolyl, (2,3)-dihydro-1H-pyrrolyl, tetrahydrothiopyranyl, tetrahydropyranyl, (3,4)-dihydro-2H-pyranyl, (3,4)-dihydro-2H-thiopyranyl, (1,2,3,6)-tetrahydropyridinyl, (1,2,3,4)-tetrahydropyridinyl, (1,2,5,6)-tetrahydropyridinyl, [1,3]-oxazinanyl, hexahydropyrimidinyl, (5,6)-dihydro-4H-pyrimidinyl, oxazolidinyl, (1,3)-dioxanyl, (1,4)-dioxanyl and (1,3)-dioxolanyl.
[1264]Suitable saturated or unsaturated heterocyclic rings which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system may preferably be selected from the group consisting of pyrrolidinyl, piperidinyl, piperazinyl, homopiperazinyl, morpholinyl, aziridinyl, azetidinyl, imidazolidinyl, thiomorpholinyl, pyrazolidinyl, azepanyl, diazepanyl, azocanyl, (1,2,3,6)-tetrahydropyridinyl, (1,2,3,4)-tetrahydropyridinyl, (1,2,5,6)-tetrahydropyridinyl, hexahydropyrimidinyl, (5,6)-dihydro-4H-pyrimidinyl, pyridazin-3(2H)-on-yl, phthalazin-1(2H)-on-yl, indolinyl, isoindolinyl, decahydronaphthyl, (1,2,3,4)-tetrahydroquinolinyl, (1,2,3,4)-tetrahydroisoquinolinyl, (1,2,3,4)-tetrahydronaphthyl, octahydro-cyclopenta[c]pyrrolyl, (1,3,4,7,9a)-hexahydro-2H-quinolizinyl, (1,2,3,5,6,8a)-hexahydro-indolizinyl, decahydroquinolinyl, octahydropyrrolo[1,2-a]pyrazinyl, octahydro-1H-pyrido[1,2-a]pyrazinyl, dodecahydrocarbazolyl, 9H-carbazolyl, decahydroisoquinolinyl and (2,3)-dihydro-1H-benzo[de]isoquinolinyl.
[1265]Suitable aromatic heterocyclic rings which may be condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system may preferably be selected from the group consisting of imidazolyl, pyrazolyl, triazolyl, (4,5,6,7)-tetrahydro-2H-indazolyl, indazolyl and benzimidazolyl.
[1266]Suitable saturated or unsaturated, optionally at least one heteroatom as ring member containing cycloaliphatic radicals, C3-9cycloalkyl radicals or C4-9cycloalkenyl radicals which are condensed with an unsubstituted or at least mono-substituted mono- or bicyclic ring system may preferably be selected from the group consisting of indolinyl, isoindolinyl, decahydronaphthyl, (1,2,3,4)-tetrahydroquinolinyl, (1,2,3,4)-tetrahydroisoquinolinyl, (1,2,3,4)-tetrahydronaphthyl, octahydro-cyclopenta[c]pyrrolyl, (1,3,4,7,9a)-hexahydro-2H-quinolizinyl, (1,2,3,5,6,8a)-hexahydro-indolizinyl, decahydroquinolinyl, dodecahydrocarbazolyl, 9H-carbazolyl, decahydroisoquinolinyl, (6,7)-dihydro-4H-thieno[3,2-c]pyridinyl, (2,3)-dihydro-1H-benzo[de] isoquinolinyl, octahydropyrrolo[1,2-a]pyrazinyl, octahydro-1H-pyrido[1,2-a]pyrazinyl, (1,2,3,7,8,8a)-hexahydroindolizinyl, (2,6,7,8,9,9a)-hexahydro-1H-quinolizinyl, octahydroindolizinyl, octahydro-1H-quinolizinyl, (1,2,3,5,8,8a)-hexahydroindolizinyl, (4,6,7,8,9,9a)-hexahydro-1H-quinolizinyl, fluorenyl and (1,2,3,4)-tetrahydroquinoxalinyl.
[1267]If any of the substituents in any of the above defined formulae represents an alkylene group, preferably an C1-6alkylene group, an alkenylene group, preferably an C2-6alkenylene group or an alkinylene group, preferably an C2-6alkinylene group, which may be substituted, said alkylene group, C2-6alkylene group, alkenylene group, C2-6alkenylene group, alkinylene group or C2-6alkinylene group may be unsubstituted or substituted by one or more substituents, preferably unsubstituted or optionally substituted with 1, 2 or 3 substituent(s). Said substituent(s) may preferably be selected independently from the group consisting of —O—C1-5-alkyl, —S—C1-5-alkyl, —F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2, —NH(C1-5-alkyl) and —N(C1-5-alkyl)2, whereby in each occurrence C1-5-alkyl may be linear or branched. An alkenylene group comprises at least one carbon-carbon double bond, an alkinylene group comprises at least one carbon-carbon triple bond.
[1268]Suitable alkylene groups include —(CH2)—, —CH(CH3)—, —CH(phenyl), —(CH2)2—, —(CH2)3—, —(CH2)4—, —(CH2)5and —(CH2)6—, suitable alkenylene groups include —CH—CH—, —CH2—CH═CH— and —CH═CH—CH2— and suitable alkinylene groups include —C≡C—, —CH2—C≡C— and —C≡C—CH2—.
[1269]If any of the substituents in any of the above defined formulae represents or comprises an aryl radical, including a 6-membered aryl radical such as phenyl or a 10-membered aryl radical such as naphthyl or a 14-membered aryl radical such as anthracenyl, said aryl radical may—if not defined otherwise—be unsubstituted or substituted by one or more substituents, preferably unsubstituted or substituted with 1, 2, 3, 4 or 5 substituent(s). Said substituent(s) may preferably be selected independently from the group consisting of C1-5-alkyl, —O—C1-5-alkyl, —S—C1-5-alkyl, —C(═O)—OH, —C(═O)—C1-5-alkyl, —C(═O)—O—C1-5-alkyl, —O—C(═O)—C1-5-alkyl, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —SH, —NH2, —NH(C1-5-alkyl), —N(C1-5-alkyl)2, —NO2, —CHO, —CF2H, —CFH2, —C(═O)—NH2, —C(═O)—NH(C1-5-alkyl), —C(═O)—N(C1-5-alkyl)2, —S(═O)2—C1-5-alkyl, —S(═O)2-phenyl, —C1-5-alkylene-C(═O)—OH, —C1-5-alkylene-C(═O)—O—C1-5-alkyl, —NH—C(═O)—C1-5-alkyl, —NH—S(═O)2—C1-5-alkyl, pyrrolidinyl, piperidinyl, morpholinyl, oxazolyl, isoxazolyl, pyridinyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, thiophenyl, furanyl, pyrrolidin-2,5-dionyl, phenyl, phenoxy and benzyl; whereby in each occurrence C1-5-alkyl may be linear or branched and whereby said cyclic substituent(s) may be unsubstituted or substituted by 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, methoxy, ethoxy, F, Cl, Br, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2and —NO2.
[1270]More preferably said substituents may be selected independently from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, n-pentyl, —O—CH3, —O—C2H5, —O—CH2—CH2—CH3, —O—CH(CH3)2, —O—C(CH3)3, —S—CH3, —S—C2H5, —S—CH2—CH2—CH3, —S—CH(CH3)2, —S—C(CH3)3, —C(═O)—OH, —C(═O)—O—CH3, —C(═O)—O—C2H5, —C(═O)—O—CH3—CH3—CH3, —C(═O)—O—CH(CH3)2, —C(═O)—O—C(CH3)3, —C(═O)—CH3, —C(═O)—C2H5, —C(═O)—CH3—CH3—CH3, —C(═O)—CH(CH3)2, —C(═O)—C(CH3)3, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2, —NH—CH3, —NH—C2H5, —NH—CH2—CH2—CH3, —NH—CH(CH3)2, —NH—C(CH3)3, —N(CH3)2, —N(C2H5)2, —NO2, —CHO, —CF2H, —CFH2, —C(═O)—NH2, —C(═O)—NH—CH3, —C(═O)—NH—C2H5, —C(═O)—N(CH3)2, —C(═O)—N(C2H5)2and —S(═O)2—CH3.
[1271]Preferred aryl radicals, which may optionally be at least mono-substituted, are phenyl and naphthyl.
[1272]Suitable aryl radicals, which are condensed with an unsubstituted or at least mono-substituted saturated or unsaturated mono- or bicyclic ring system, may preferably be selected from the group consisting of indolinyl, isoindolinyl, (1,2,3,4)-tetrahydroquinolinyl, (1,2,3,4)-tetrahydroisoquinolinyl, (1,2,3,4)-tetrahydronaphthyl, (1,2,3,4)-tetrahydroquinoxalinyl, benzo[d]oxazol-2(3H)-onyl, benzo[d]thiazol-2(3H)-onyl, 2,3-dihydrobenzo[b][1,4]dioxinyl, benzo[d][1,3]dioxolyl, 3,4-dihydro-2H-benzo[b][1,4]oxazinyl, isochromanyl, chromanyl, 2,3-dihydrobenzofuranyl and 1H-benzo[b][1,4]diazepine-2,4(3H,5H)-dionyl.
[1273]If any of the substituents in any of the above defined formulae represents or comprises a heteroaryl radical, including a monocyclic 5- or 6-membered heteroaryl radical or a bi- or tricyclic 8-, 9-, 10-, 11-, 12-, 13- or 14 membered heteroaryl radical, said heteroaryl radical may—if not defined otherwise—be unsubstituted or substituted by one or more substituents, preferably unsubstituted or substituted with 1, 2, 3, 4 or 5 substituent(s). Said substituent(s) may preferably be selected independently from the group consisting of C1-5-alkyl, —O—C1-5-alkyl, —S—C1-5-alkyl, —C(═O)—OH, —C(═O)—C1-5-alkyl, —C(═O)—O—C1-5-alkyl, —O—C(═O)—Cl—5-alkyl, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —SH, —NH2, —NH(C1-5-alkyl), —N(C1-5-alkyl)2, —NO2, —CHO, —CF2H, —CFH2, —C(═O)—NH2, —C(═O)—NH(C1-5-alkyl), —C(═O)—N(C1-5-alkyl)2, —S(═O)2—C1-5-alkyl, —S(═O)2-phenyl, —C1-5-alkylene-C(═O)—OH, —C1-5-alkylene-C(═O)—O—C1-5-alkyl, —NH—C(═O)—C1-5-alkyl, S(═O)2—C1-5-alkyl, pyrrolidinyl, piperidinyl, morpholinyl, oxazolyl, isoxazolyl, pyridinyl, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, thiophenyl, furanyl, pyrrolidin-2,5-dionyl, phenyl, phenoxy and benzyl; whereby in each occurrence C1-5-alkyl may be linear or branched and whereby said cyclic substituent(s) may be unsubstituted or substituted by 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, methoxy, ethoxy, F, Cl, Br, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2and —NO2
[1274]More preferably said substituents may be selected independently from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, n-pentyl, —O—CH3, —O—C2H5, —O—CH2—CH2—CH3, —O—CH(CH3)2, —O—C(CH3)3, —S—CH3, —S—C2H5, —S—CH2—CH2—CH3, —S—CH(CH3)2, —S—C(CH3)3, —C(═O)—OH, —C(═O)—O—CH3, —C(═O)—O—C2H5, —C(═O)—O—CH3—CH3—CH3, —C(═O)—O—CH(CH3)2, —C(═O)—O—C(CH3)3, —C(═O)—CH3, —C(═O)—C2H5, —C(═O)—CH3—CH3—CH3, —C(═O)—CH(CH3)2, —C(═O)—C(CH3)3, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2, —NH—CH3, —NH—C2H5, —NH—CH2—CH2—CH3, —NH—CH(CH3)2, —NH—C(CH3)3, —N(CH3)2, —N(C2H5)2, —NO2, —CHO, —CF2H, —CFH2, —C(═O)—NH2, —C(═O)—NH—CH3, —C(═O)—NH—C2H5, —C(═O)—N(CH3)2, —C(═O)—N(C2H5)2and —S(═O)2—CH3.
[1275]The heteroatom(s), which are present as ring member(s) in the heteroaryl radical, may, unless defined otherwise, independently be selected from the group consisting of nitrogen, oxygen and sulphur. Preferably the heteroaryl radical comprises 1, 2, 3 or 4 heteroatom(s).
[1276]Suitable bi- or tricyclic heteroaryl radicals, which may optionally be at least mono-substituted, may preferably be selected from the group consisting of indolyl, isoindolyl, quinolinyl, isoquinolinyl, benzo[b]furanyl, benzo[b]thiophenyl, benzimidazolyl, benzo[2,1,3]thiadiazolyl, [1,2,3]-benzothiadiazolyl, [2,1,3]-benzoxadiazolyl, [1,2,3]-benzoxadiazolyl, benzoxazolyl, benzothiazolyl, benzisoxazolyl, benzisothiazolyl, imidazo[2,1-b]thiazolyl, 2H-chromenyl, indazolyl and quinazolinyl.
[1277]Suitable mono-, bi- or tricyclic heteroaryl radicals, which are condensed with an unsubstituted or at least mono-substituted saturated or unsaturated mono- or bicyclic ring system, may preferably be selected from the group consisting of [1,3]-benzodioxolyl, [1,4]-benzodioxanyl, [1,2,3,4]-tetrahydronaphthyl, (2,3)-dihydro-1H-cyclopenta[b]indolyl, [1,2,3,4]-tetrahydroquinolinyl, [1,2,3,4]-tetrahydroisoquinolinyl, [1,2,3,4]-tetrahydroquinazolinyl and [3,4]-dihydro-2H-benzo[1,4]oxazinyl.
[1278]Suitable monocyclic heteroaryl radicals, which may optionally be at least mono-substituted, may preferably be selected from the group consisting of pyridinyl, furyl (furanyl), thiophenyl (thiophenyl), pyrrolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, imidazolyl, pyrazolyl, oxadiazolyl, thiadiazolyl, triazolyl, pyridazinyl, pyrimidinyl, pyrazinyl and pyranyl.
[1279]A mono- or bicyclic ring system according to the present invention—if not defined otherwise—means a mono- or bicyclic hydrocarbon ring system that may be saturated, unsaturated or aromatic. Each of its different rings may show a different degree of saturation, i.e. it may be saturated, unsaturated or aromatic. Optionally each of the rings of the mono- or bicyclic ring system may contain one or more, preferably 1, 2 or 3, heteroatom(s) as ring member(s), which may be identical or different and which can preferably be selected from the group consisting of N, O and S. The rings of the mono- or bicyclic ring system are preferably 5-, 6- or 7-membered.
[1280]Preferably a mono- or bicyclic ring system according to the present invention is a phenyl or naphthyl ring system.
[1281]The term “condensed” according to the present invention means that a ring or ring system is attached to another ring or ring system, whereby the terms “annulated” or “annelated” are also used by those skilled in the art to designate this kind of attachment.
[1282]Such a mono- or bicyclic ring system may—if not defined otherwise—be unsubstituted or substituted by one or more substituents, preferably unsubstituted or substituted with 1, 2, 3, 4 or 5 substituent(s). Said substituents may preferably be selected independently from the group consisting of C1-5-alkyl, —O—C1-5-alkyl, —S—C1-5-alkyl, —C(═O)—OH, oxo (═O), thioxo (═S), —C(═O)—O—C1-5-alkyl, —O—C(═O)—C1-5-alkyl, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2, —NH(C1-5-alkyl), —N(C1-5-alkyl)2, —NO2, —CHO, —CF2H, —CFH2, —C(═O)—NH2, —C(═O)—NH(C1-5-alkyl), —C(═O)—N(C1-5-alkyl)2, —S(═O)2—C1-5-alkyl, —S(═O)2-phenyl, phenyl, phenoxy and benzyl; whereby in each occurrence Cl—5-alkyl may be linear or branched and whereby said cyclic substituents may be unsubstituted or substituted by 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, methoxy, ethoxy, F, Cl, Br, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2and —NO2.
[1283]More preferably said substituents may be selected from the group consisting of oxo (═O), thioxo (═S), methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, sec-butyl, isobutyl, n-pentyl, —O—CH3, —O—C2H5, —O—CH2—CH2—CH3, —O—CH(CH3)2, —O—C(CH3)3, —S—CH3, —S—C2H5, —S—CH2—CH2—CH3, —S—CH(CH3)2, —S—C(CH3)3, —C(═O)—OH, —C(═O)—O—CH3, —C(═O)—O—C2H5, —C(═O)—O—CH3—CH3—CH3, —C(═O)—O—CH(CH3)2, —C(═O)—O—C(CH3)3, —C(═O)—CH3, —C(═O)—C2H5, —C(═O)—CH3—CH3—CH3, —C(═O)—CH(CH3)2, —C(═O)—C(CH3)3, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2, —NH—CH3, —NH—C2H5, —NH—CH2—CH2—CH3, —NH—CH(CH3)2, —NH—C(CH3)3, —N(CH3)2, —N(C2H5)2, —NO2, —CHO, —CF2H, —CFH2, —C(═O)—NH2, —C(═O)—NH—CH3, —C(═O)—NH—C2H5, —C(═O)—N(CH3)2, —C(═O)—N(C2H5)2, —S(═O)2—CH3, —S(═O)2-phenyl, phenyl, phenoxy and benzyl; whereby in each occurrence said cyclic substituents may be unsubstituted or substituted by 1, 2 or 3 substituent(s) independently selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, methoxy, ethoxy, F, Cl, Br, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2and —NO2.
[1284]If any of the substituents in any of the above defined formulae represents a saturated or unsaturated aliphatic radical, i.e. an alkyl radical, preferably an C1-10alkyl radical; an alkenyl radical, preferably an C2-10alkenyl radical or an alkinyl radical, preferably an C2-10alkinyl radical; said aliphatic radical may—if not defined otherwise—be unsubstituted or substituted by one or more substituents, preferably unsubstituted or substituted with 1, 2, 3, 4 or 5 substituent(s). Said substituent(s) may preferably be selected independently from the group consisting of —O—C1-5-alkyl, —S—C1-5-alkyl, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2, —NH(C1-5-alkyl) and —N(C1-5-alkyl)2, whereby in each occurrence C1-5-alkyl may be linear or branched. More preferably said substituent(s) may preferably be selected independently from the group consisting of —O—CH3, —O—C2H5, —O—CH2—CH2—CH3, —O—CH(CH3)2, —O—C(CH3)3, —S—CH3, —S—C2H5, —S—CH2—CH2—CH3, —S—CH(CH3)2, —S—C(CH3)3, F, Cl, Br, I, —CN, —CF3, —OCF3, —SCF3, —OH, —SH, —NH2, NH—CH3, —NH—C2H5, —NH—CH2—CH2—CH3, —NH—CH(CH3)2, —NH—C(CH3)3, —N(CH3)2, —N(C2H5)2.
[1285]An alkenyl radical comprises at least one carbon-carbon double bond, an alkinyl radical comprises at least one carbon-carbon triple bond.
[1286]Suitable alkyl radicals, which may be substituted by one or more substituents, may preferably be selected from the group consisting of methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, n-hexyl, n-heptyl, n-octyl, n-nonyl and n-decyl.
[1287]Suitable alkenyl radicals, which may be substituted by one or more substituents, may preferably be selected from the group consisting of vinyl, 1-propenyl, 2-propenyl, 1-butenyl, 2-butenyl and 3-butenyl.
[1288]Suitable alkinyl radicals, which may be substituted by one or more substituents, may preferably be selected from the group consisting of ethinyl, 1-propinyl, 2-propinyl, 1-butinyl, 2-butinyl and 3-butinyl.
[1289]The NMDA receptor is a cell-surface protein complex, widely distributed in the mammalian central nervous system that belongs to the class of ionotropic-glutamate receptors. It is involved in excitatory-synaptic transmission and the regulation of neuronal growth. The structure comprises a ligand-gated/voltage-sensitive ion channel. The NMDA receptor is highly complex and is believed to contain at least five distinct binding (activation) sites: a glycine-binding site, a glutamate-binding site (NMDA-binding site); a PCP-binding site, a polyamine-binding site, and a zinc-binding site. In general, a receptor antagonist is a molecule that blocks or reduces the ability of an agonist to activate the receptor. As used herein, an “NMDA-receptor antagonist” means any compound or composition, known or to be discovered, that when contacted with an NMDA receptor in vivo or in vitro, inhibits the flow of ions through the NMDA-receptor ion channel.
[1290]According to the present invention the meaning of the phrase “NMDA-receptor antagonist” encompasses any compound or composition that antagonizes the NMDA receptor by binding at the glycine site. Glycine-site NMDA-receptor antagonists can be identified by standard in vitro and in vivo assays. See, for example, the assays described in U.S. Pat. No. 6,251,903 (issued Jun. 26, 2001); U.S. Pat. No. 6,191,165 (issued Feb. 20, 2001); Grimwood et al. MOLECULAR PHARMACOLOGY 923 (1992); Yoneda et al. J. NEUROCHEM. 102 (1994); and Mayer et al. J. NEUROPHYSIOL. 645 (1988).
[1291]According to the present invention the meaning of the phrase “NMDA-receptor antagonist” encompasses any compound or composition that antagonizes the NMDA receptor by binding at the glutamate site. References that disclose NMDA-receptor antagonists as well as assays for identifying competitive NMDA-receptor antagonists include Jia-He Li, et al., J. MED. CHEM. 1955 (1995); Steinberg et al., NEUROSCI. LETT. 225 (1991); Meldrum et al., TRENDS PHARMACOL. SCI., 379 (1990); Willetts et al., TRENDS PHARMACOL. SCI. 423 (1990); Faden et al., TRENDS PHARMACOL. SCI. 29 (1992); Rogawski TRENDS PHARMACOL. SCI. 325 (1993); Albers et al., CLINICAL NEUROPHARM. 509 (1992); Wolfe et al., AM. J EMERG. MED., 174 (1995); and Bigge, BIOCHEM. PHARMACOL. 1547 (1993).
[1292]According to the present invention the meaning of the phrase “NMDA-receptor antagonist” encompasses any compound or composition that antagonizes the NMDA receptor by binding at the PCP (phencyclidine) site. Non-competitive NMDA-receptor antagonists can be identified using routine assays, for example, those described in U.S. Pat. Nos. 6,251,948 (issued Jun. 26, 2001); 5,985,586 (issued Nov. 16, 1999), and 6,025,369 (issued Feb. 15, 2000); Jacobson et al., J. PHARMACOL. EXP. THER. 243 (1987); and Thurkauf et al., J. MED. CHEM. 2257 (1988).
[1293]According to the present invention the meaning of “NMDA-receptor antagonist” encompasses compounds that block the NMDA receptor at the polyamine binding site, the zinc-binding site, and other NMDA-receptor antagonists that are either not classified herein according to a particular binding site or that block the NMDA receptor by another mechanism. Examples of NMDA-receptor antagonists that bind at the polyamine site include, but are not limited to, sperrine, spermidine, putrescine, and arcaine. Examples of assays useful to identify NMDA-receptor antagonists that act at the zinc or polyamine binding site are disclosed in U.S. Pat. No. 5,834,465 (issued Nov. 10, 1998).
[1294]NMDA-receptor antagonists for use in the present invention include 3-((−)-2-carboxypiperazin-4-ylpropyl-1-phosphate (CPP); 3-(2-carboxypiperazin-4-yl)-prop enyl-1-phosphonate (CPP-ene); 1-(cis-2-carboxypiperidine-4-yl)methyl-1-phosphonic acid (CGS 19755); D-2-Amino-5-phosphonopentanoic acid (AP5); 2-amino-phosphonoheptanoate (AP7); D,L-(E)-2-amino-4-methyl-5-phosphono-3-pentenoic acid carboxyethyl ester (CGP39551); 2-amino-4-methyl-5-phosphono-pent-3-enoic acid (CGP 40116); (4-phosphono-but-2-enylamino)-acetic acid (PD 132477); 2-amino-4-oxo-5-phosphono-pentanoic acid (MDL 100,453); 3-((phosphonylmethyl)-sulfinyl)-D,L-alanine; amino-(4-phosphonomethyl-phenyl)-acetic acid (PD 129635); 2-amino-3-(5-chloro-1-phosphonomethyl-1H-benzoimidazol-2-yl)-propionic acid; 2-amino-3-(3-phosphonomethyl-quinoxalin-2-yl)-propionic acid; 2-amino-3-(5-phosphonomethyl-biphenyl-3-yl)-propionic acid (SDZ EAB 515); 2-amino-3-[2-(2-phosphono-ethyl)-cyclohexyl]-propionic acid (NPC 17742); 4-(3-phosphono-propyl)-piperazine-2-carboxylic acid (D-CPP); 4-(3-phosphono-allyl)-piperazine-2-carboxylic acid (D-CPP-ene); 4-phosphonomethyl-piperidine-2-carboxylic acid (CGS 19755); 3-(2-phosphono-acetyl)-piperidine-2-carboxylic acid (MDL 100,925); 5-phosphono-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid (SC 48981); 5-(2-phosphono-ethyl)-1,2,3,4-tetrahydro-isoquinoline-3-carboxylic acid (PD 145950); 6-phosphonomethyl-decahydro-isoquinoline-3-carboxylic acid (LY 274614); 4-(1H-tetrazol-5-ylmethyl)-piperidine-2-carboxylic acid (LY 233053 and 235723); 6-(1H-Tetrazol-5-ylmethyl)-decahydro-isoquinoline-3-carboxylic acid (LY 233536); ketamine; phencyclidine; dextromethorphan; dextrorphan; dexoxadrol; dizocilpine (MK-801); remacemide; thienylcyclohexylpiperidine (TCP); N-allylnometazocine (SKF 10,047); cyclazocine; etoxadrol; (1,2,3,4,9,9a-hexahydro-fluoren-4a-yl)-m-ethyl-amine (PD 137889); (1,3,4,9,10,10a-hexahydro-2H-phenanthren-4a-yl)-methyl-amine (PD 138289); PD 138558; tiletamine; kynurenic acid; 7-chloro-kynurenic acid; memantine; nitromemantine; quinoxalinediones such as 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 6,7-dinitro-quinoxaline-2-,3-dione (DNQX); amantadine; eliprodil; iamotrigine; riluzole; aptiganel; flupirtine; celfotel; levemopamil; 1-(4-hydroxy-phenyl)-2-(4-phenylsulfanyl-piperidin-1-yl)-propan-1-one; 2-[4-(4-fluoro-benzoyl)-piperidin-1-yl]-1-naphthalen-2-yl-ethanone (E 2001); 3-(1,1-dimethyl-heptyl)-9-hydroxymethyl-6,6-dimethyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol (HU-211); 1-{4-[1-(4-chloro-phenyl)-1-methyl-ethyl]-2-methoxy-phenyl}-1H-[1,2,4]triazole-3-carboxylic acid amide (CGP 31358); acetic acid 10-hydroxy-7,9,7′,9′-tetramethoxy-3,3′-dimethyl-3,4,3′,4′-tetrahydro-1H,1′H-[5,5′]bi[benzo[g]isochromenyl]-4-yl ester (ES 242-1); 14-hydroxy-11-isopropyl-10-methyl-5-octyl-10,13-diaza-tricyclo[6.6.1.04,1-5]pentadeca-1,4,6,8(15)-tetraen-12-one; and 4,5-dioxo-4,5-dihydro-1H-benzo-[g]indole-2,7,9-tricarboxylic acid (PQQ).
[1295]Particularly preferably memantine is present as component (B). Memantine (CAS Registry No. 41100-52-1), is an uncompetitive N-methyl-D-aspartate antagonist currently used for the treatment of dementia syndrome, spinal spasticity and Parkinson's disease. Chemically, memantine is 1-amino-3,5-dimethyladamantane of the adamantine class.
[1296]Further derivatives of memantine and nitromemantine can be prepared as outlined in U.S. patent application 2004/0122090.
[1297]The term “cognitive disorder” indicates disruptions in performance including one or more of the following signs:
[0000]1) memory deficits (impaired ability to learn new information or recall previously learned information;
2) one (or more) of the following disturbances:
a) aphasia (language disturbance)
b) apraxia (impaired ability to carry out motor activities despite intact motor function)
c) agnosia (failure to recognize or identify objects despite in tact sensory function)
d) disturbance in executive functioning (i.e. planning, organizing, sequencing, abstracting);
3) memory disturbances causing significant impairment in social or occupational functioning, and representing a significant decline from a previous level of functioning; and
4) impairment in cognitive functioning as evidenced by neuropsychological testing or quantified clinical assessment, accompanied by objective evidence of a systemic general medical condition or central nervous system dysfunction.
[1298]Cognitive disorders (or memory disorders) may include Alzheimer's disease, senile dementia process, learning disabilities caused by degenerative disorders, learning disabilities caused by non-degenerative disorders, memory or cognitive dysfunction such as mild cognitive impairment, age-related cognitive decline, cerebral senility, vascular dementia, AIDS-associated dementia, electric shock induced amnesia, memory impairment associated with depression or anxiety, cognitive defects in Parkinson's disease, Down's syndrome, stroke, traumatic brain injury, Huntington's disease, and attention deficit disorder; especially ADHD (attention deficit/hyperactivity disorder).
[1299]“Treatment” (e.g. of cognitive disorders, e.g. depression or e.g. of obesity and obesity-related disorders) refers to the administration of the compounds or combinations of the present invention to treat (in the case of cognitive disorders or depression) the disorder or—more often—the symptoms of these disorders; or (for obesity) reduce or maintain the body weight of an obese subject. One outcome of treatment may be ameliorating the symptoms of e.g. Alzheimer's disease or the clinical depression or may be reducing the body weight of an obese subject relative to that subject's body weight immediately before the administration of the compounds or combinations of the present invention. A preferred aspect of the treatment, especially for cognitive disorders or depression, also involves the prophylaxis against the disorder, thus preventing the occurrence of its symptoms. Another outcome of treatment may be preventing body weight regain of body weight previously lost as a result of diet, exercise, or pharmacotherapy.
[1300]Another outcome of treatment may be decreasing the occurrence of and/or the severity of obesity-related diseases. Another outcome of treatment may be to maintain weight loss. The treatment may suitably result in a reduction in food or calorie intake by the subject, including a reduction in total food intake, or a reduction of intake of specific components of the diet such as carbohydrates or fats; and/or the inhibition of nutrient absorption; and/or the inhibition of the reduction of metabolic rate; and in weight reduction in patients in need thereof. The treatment may also result in an alteration of metabolic rate, such as an increase in metabolic rate, rather than or in addition to an inhibition of the reduction of metabolic rate; and/or in minimization of the metabolic resistance that normally results from weight loss.
[1301]The term “depression” or especially “clinical depression” is referring to a state of sadness, melancholia or despair that has advanced to the point of being disruptive to an individual's social functioning and/or activities of daily living.
[1302]“Obesity” is a condition in which there is an excess of body fat. The operational definition of obesity is based on the Body Mass Index (BMI), which is calculated as body weight per height in meters squared (kg/m2). “Obesity” refers to a condition whereby an otherwise healthy subject has a Body Mass Index (BMI) greater than or equal to 30 kg/m2, or a condition whereby a subject with at least one co-morbidity has a BMI greater than or equal to 27 kg/m2. An “obese subject” is an otherwise healthy subject with a Body Mass Index (BMI) greater than or equal to 30 kg/m2or a subject with at least one co-morbidity with a BMI greater than or equal to 27 kg/m2. A “subject at risk of obesity” is an otherwise healthy subject with a BMI of 25 kg/m2to less than 30 kg/m2or a subject with at least one co-morbidity with a BMI of 25 kg/m2to less than 27 kg/m2.
[1303]The increased risks associated with obesity occur at a lower Body Mass Index (BMI) in Asians. In Asian countries, including Japan, “obesity” refers to a condition whereby a subject with at least one obesity-induced or obesity-related co-morbidity, that requires weight reduction or that would be improved by weight reduction, has a BMI greater than or equal to 25 kg/m2. In Asian countries, including Japan, an “obese subject” refers to a subject with at least one obesity-induced or obesity-related co-morbidity that requires weight reduction or that would be improved by weight reduction, with a BMI greater than or equal to 25 kg/m2. In Asia-Pacific, a “subject at risk of obesity” is a subject with a BMI of greater than 23 kg/m2to less than 25 kg/m2.
[1304]As used herein, the term “obesity” is meant to encompass all of the above definitions of obesity.
[1305]Obesity-induced or obesity-related co-morbidities include, but are not limited to, diabetes, non-insulin dependent diabetes mellitus-type II (2), impaired glucose tolerance, impaired fasting glucose, insulin resistance syndrome, dyslipidemia, hypertension, hyperuricacidemia, gout, coronary artery disease, myocardial infarction, angina pectoris, sleep apnea syndrome, Pickwickian syndrome, fatty liver; cerebral infarction, cerebral thrombosis, transient ischemic attack, orthopedic disorders, arthritis deformans, lumbodynia, emmeniopathy, and infertility.
[1306]In particular, co-morbidities include: hypertension, hyperlipidemia, dyslipidemia, glucose intolerance, cardiovascular disease, sleep apnea, diabetes mellitus, and other obesity-related conditions.
[1307]The term “Metabolic syndrome”, also known as syndrome X, is defined in the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (ATP-E). E. S. Ford et al., JAMA, Vol. 287 (3), Jan. 16, 2002, pp 356-359. Briefly, a person is defined as having Metabolic syndrome if the person has three or more of the following symptoms: abdominal obesity, hypertriglyceridemia, low HDL cholesterol, high blood pressure, and high fasting plasma glucose.
[1308]The terms “administration of” and or “administering a” compound should be understood to mean providing a compound of the invention or a prodrug of a compound of the invention to a subject in need of treatment.
[1309]The instant pharmaceutical composition includes administration of a single pharmaceutical dosage formulation which contains both the compound with 5-HT6receptor affinity, and at least one NMDA-receptor ligand, as well as administration of each active agent in its own separate pharmaceutical dosage formulation. Where separate dosage formulations are used, the individual components of the composition can be administered at essentially the same time, i.e., concurrently, or at separately staggered times, i.e. sequentially prior to or subsequent to the administration of the other component of the composition. The instant pharmaceutical composition is therefore to be understood to include all such regimes of simultaneous or alternating treatment, and the terms “administration” and “administering” are to be interpreted accordingly.
[1310]Administration in these various ways are suitable for the present compositions as long as the beneficial pharmaceutical effect of the combination of the compound with 5-HT6receptor affinity, and at least one NMDA-receptor ligand, is realised by the patient at substantially the same time.
[1311]Such beneficial effect is preferably achieved when the target blood level concentrations of each active drug are maintained at substantially the same time. It is preferred that the combination of the compound with 5-HT6receptor affinity, and at least one NMDA-receptor ligand, be co-administered concurrently on a once-a-day dosing schedule; however, varying dosing schedules, such as the compound with 5-HT6receptor affinity once a day and the NMDA-receptor ligand once, twice or more times per day, is also encompassed herein. A single oral dosage formulation comprised of both a compound with 5-HT6receptor affinity and a NMDA-receptor ligand is preferred. A single dosage formulation will provide convenience for the patient, which is an important consideration especially for patients with diabetes or obese patients who may be in need of multiple medications.
[1312]The term “subject” as used herein refers to an animal, preferably a mammal, most preferably a human, who has been the object of treatment, observation or experiment.
[1313]The administration of the composition of the present invention in order to practice the present methods of therapy is carried out by administering a therapeutically effective amount of the compounds in the composition to a subject in need of such treatment or prophylaxis. The need for a prophylactic administration according to the methods of the present invention is determined via the use of well known risk factors. The effective amount of an individual compound is determined, in the final analysis, by the physician in charge of the case, but depends on factors such as the exact disease to be treated, the severity of the disease and other diseases or conditions from which the patient suffers, the chosen route of administration, other drugs and treatments which the patient may concomitantly require, and other factors in the physician's judgement.
[1314]The term “therapeutically effective amount” as used herein means the amount of the active compounds in the composition that will elicit the biological or medical response in a tissue, system, subject, or human that is being sought by the researcher, veterinarian, medical doctor or other clinician, which includes alleviation of the symptoms of the disorder being treated. The novel methods of treatment of this invention are for disorders known to those skilled in the art.
[1315]The term “salt” as used herein is to be understood as meaning any form of the compounds in which they assume an ionic form or are charged and are coupled with a counter-ion (a cation or anion) or are in solution. By this are also to be understood complexes of the active compound with other molecules and ions, in particular complexes which are complexed via ionic interactions.
[1316]The term “physiologically acceptable salt” is understood in particular, in the context of this invention, as salt (as defined above) formed either with a physiologically tolerated acid, that is to say salts of the particular active compound with inorganic or organic acids which are physiologically tolerated—especially if used on humans and/or mammals—or with at least one, preferably inorganic, cation which are physiologically tolerated—especially if used on humans and/or mammals. Examples of physiologically tolerated salts of particular acids are salts of: hydrochloric acid, hydrobromic acid, sulfuric acid, hydrobromide, monohydrobromide, monohydrochloride or hydrochloride, methiodide, methanesulfonic acid, formic acid, acetic acid, oxalic acid, succinic acid, malic acid, tartaric acid, mandelic acid, fumaric acid, lactic acid, citric acid, glutamic acid, hippuric acid, picric acid and/or aspartic acid. Examples of physiologically tolerated salts of particular bases are salts of alkali metals and alkaline earth metals and with NH4.
[1317]Solvates, preferably hydrates, of the compounds of the present invention and in each case of corresponding stereoisomers may also be obtained by standard procedures known to those skilled in the art.
[1318]The term “solvate” according to this invention is to be understood as meaning any form of the compounds in which they have attached to it via non-covalent binding another molecule (most likely a polar solvent) especially including hydrates and alcoholates, e.g. methanolate.
[1319]The active substance combination according to this invention comprises preferably 1-99% by weight of the component (A) and 99-1% by weight of the component (B), more preferably 10-80% by weight of the component (A) and 80-20% by weight of the component (B), these percentages referring to the total weight of both components (A) and (B).
[1320]Another aspect of the present invention is a medicament, which comprises an inventive active substance combination and optionally one or more pharmacologically acceptable adjuvants.
[1321]Said medicament is suitable for simultaneous NMDA-receptor inhibition and 5-HT6-receptor regulation.
[1322]Said medicament is particularly preferably suitable for the prophylaxis and/or treatment of disorders related to food ingestion, preferably for prophylaxis and/or treatment of obesity, anorexia, cachexia, bulimia, diabetes, preferably type II diabetes (non-insulin-dependent diabetes mellitus), or for prophylaxis and/or treatment of gastrointestinal tract disorders, preferably of the irritable bowel syndrome, for prophylaxis and/or treatment of Metabolic Syndrome, Peripheral Nervous System Disorders, Central Nervous System Disorders, arthritis, epilepsy, anxiety, panic, depression, cognitive disorders, memory disorders, cardiovascular diseases, senile dementia processes, such as Alzheimer's, Parkinson's and/or Huntington's Disease, schizophrenia, psychosis, infantile hyperkinesia (ADHD, attention deficit/hyperactivity disorder), pain, hypertensive syndrome, inflammatory diseases, immunologic diseases or for improvement of cognition.
[1323]Said medicament is more particularly preferably suitable for the prophylaxis and/or treatment of cognitive disorders or memory disorders like Alzheimer's disease, senile dementia process, learning disabilities caused by degenerative disorders, learning disabilities caused by non-degenerative disorders, memory or cognitive dysfunction such as mild cognitive impairment, age-related cognitive decline, cerebral senility, vascular dementia, AIDS-associated dementia, electric shock induced amnesia, memory impairment associated with depression or anxiety, cognitive defects in Parkinson's disease, Down's syndrome, stroke, traumatic brain injury, Huntington's disease, and attention deficit disorder; especially ADHD (attention deficit/hyperactivity disorder). Said medicament is more particularly preferably also suitable for the prophylaxis and/or treatment of depression as well as anxiety and panic.
[1324]Said medicament is more particularly preferably also suitable for the regulation of appetite, for maintenance, increase or reduction of body weight, for prophylaxis and/or treatment of disorders related to food ingestion, preferably for prophylaxis and/or treatment of obesity, anorexia, cachexia, bulimia, diabetes, preferably type II diabetes (non-insulin-dependent diabetes mellitus), or for prophylaxis and/or treatment of gastrointestinal tract disorders, preferably of the irritable bowel syndrome.
[1325]Said medicament is even more particularly preferably suitable for the prophylaxis and/or treatment of obesity.
[1326]Said medicament is also particularly preferably suitable for the prophylaxis and/or treatment of obesity-related disorders such as elevated plasma insulin concentrations and insulin resistance, dyslipidemias, hyperlipidemia, endometrial, breast, prostate and colon cancer, osteoarthritis, obstructive sleep apnea, cholelithiasis, gallstones, heart disease, abnormal heart rhythms and arrythmias, myocardial infarction, congestive heart failure, coronary heart disease, sudden death, stroke, polycystic ovary disease, craniopharyngioma, the Prader-Willi Syndrome and Frohlich's syndrome. Further examples of obesity-related disorders are reproductive hormone abnormalities, sexual and reproductive dysfunction, such as impaired fertility, infertility, hypogonadism in males and hirsutism in females, fetal defects associated with maternal obesity, gastrointestinal motility disorders, such as obesity-related gastro-esophageal reflux, respiratory disorders, such as obesity-related hypoventilation syndrome (Pickwickian syndrome), breathlessness, cardiovascular disorders, inflammation, such as systemic inflammation of the vasculature, arteriosclerosis, hypercholesterolemia, hyperuricaemia, lower back pain, gallbladder disease, gout, kidney cancer, and increased anesthetic risk.
[1327]Another aspect of the present invention is the use of an inventive active substance combination for the manufacture of a medicament for simultaneous NMDA-receptor inhibition and 5-HT6-receptor regulation.
[1328]Another aspect of the present invention is the use of an inventive active substance combination for the manufacture of a medicament for the regulation of appetite, for maintenance, increase or reduction of body weight, for prophylaxis and/or treatment of disorders related to food ingestion, preferably for prophylaxis and/or treatment of obesity, anorexia, cachexia, bulimia, diabetes, preferably type II diabetes (non-insulin-dependent diabetes mellitus), or for prophylaxis and/or treatment of gastrointestinal tract disorders, preferably of the irritable bowel syndrome, for prophylaxis and/or treatment of Peripheral Nervous System Disorders, Central Nervous System Disorders, arthritis, epilepsy, anxiety, panic, depression, preferably bipolar disorders, cognitive disorders, memory disorders, cardiovascular diseases, senile dementia processes, neurodegenerative disorders, preferably Alzheimer's disease, Parkinson's disease, Huntington's Disease and/or multiple sclerosis, schizophrenia, psychosis, infantile hyperkinesia (ADHD, attention deficit/hyperactivity disorder), pain, hypertensive syndrome, inflammatoric diseases, immunologic diseases or for improvement of cognition.
[1329]Particularly preferred is the use of an inventive active substance combination for the manufacture of a medicament for the prophylaxis and/or treatment of cognitive disorders or memory disorders like Alzheimer's disease, senile dementia process, learning disabilities caused by degenerative disorders, learning disabilities caused by non-degenerative disorders, memory or cognitive dysfunction such as mild cognitive impairment, age-related cognitive decline, cerebral senility, vascular dementia, AIDS-associated dementia, electric shock induced amnesia, memory impairment associated with depression or anxiety, cognitive defects in Parkinson's disease, Down's syndrome, stroke, traumatic brain injury, Huntington's disease, and attention deficit disorder; especially ADHD (attention deficit/hyperactivity disorder).
[1330]Also particularly preferred is the use of an inventive active substance combination for the manufacture of a medicament for the prophylaxis and/or treatment of depression as well as anxiety and panic.
[1331]Also particularly preferred is the use of an inventive active substance combination for the manufacture of a medicament for the regulation of appetite, for maintenance, increase or reduction of body weight, for prophylaxis and/or treatment of disorders related to food ingestion, preferably for prophylaxis and/or treatment of obesity, anorexia, cachexia, bulimia, diabetes, preferably type II diabetes (non-insulin-dependent diabetes mellitus), or for prophylaxis and/or treatment of gastrointestinal tract disorders, preferably of the irritable bowel syndrome.
[1332]Those skilled in the art understand that the components (A) and (B) of the active substance combination according to the present invention may be administered simultaneously or sequentially to one another, whereby in each case components (A) and (B) may be administered via the same or different administration pathways, e.g. orally or parentally. preferably both components (A) and (B) are administered simultaneously in one and the same administration form.
[1333]Yet another aspect of the present invention are pharmaceutical formulations in different pharmaceutical forms comprising an inventive active substance combination and optionally one or more pharmacologically acceptable adjuvants.
[1334]As well known to somebody skilled in the art the pharmaceutical formulations may—depending on their route of administration, also contain one or more auxiliary substances known to those skilled in the art.
[1335]The pharmaceutical formulations according to the present invention may be produced according to standard procedures known to those skilled in the art, e.g. from the tables of contents from “Pharmaceutics: the Science of Dosage Forms”, Second Edition, Aulton, M. E. (Ed.) Churchill Livingstone, Edinburgh (2002); “Encyclopedia of Pharmaceutical Technology”, Second Edition, Swarbrick, J. and Boylan J. C. (Eds.), Marcel Dekker, Inc. New York (2002); “Modern Pharmaceutics”, Fourth Edition, Banker G. S. and Rhodes C. T. (Eds.) Marcel Dekker, Inc. New York 2002 and “The Theory and Practice of Industrial Pharmacy”, Lachman L., Lieberman H. and Kanig J. (Eds.), Lea & Febiger, Philadelphia (1986). The respective descriptions are incorporated by reference and are part of the disclosure.
[1336]Preferred pharmaceutical formulations are solid pharmaceutical forms, preferably tablets, chewing tablets, chewing gums, dragées, capsules, suppositories, powder preparations, transdermal therapeutic systems, transmucosal therapeutic systems, preferably tablets or capsules.
[1337]Preferred pharmaceutical formulations are also liquid and semi-liquid pharmaceutical forms such as drops or such as juice, syrup, solution, emulsion, suspension, preferably drops or solutions.
[1338]In an additional preferred embodiment, the pharmaceutical formulations are in the form of multiparticulates, preferably microtablets, microcapsules, microspheroids, granules, crystals or pellets, optionally compacted in a tablet, filled in a capsule or suspended in a suitable liquid.
[1339]The pharmaceutical formulations according to the present invention are particularly preferably suitable for oral, intravenous, intramuscular, subcutaneous, intrathecal, epidural, buccal, sublingual, pulmonal, rectal, transdermal, nasal or intracerebroventricular application, more particularly for oral, intravenous or intraperitoneal application.
[1340]In one embodiment of the present invention the pharmaceutical formulation comprises at least one of the components (A) and (B) of the active substance combination at least partially in a sustained-release form.
[1341]By incorporating one or both of these components (A) and (B) at least partially or completely in a sustained-release form it is possible to extend the duration of their effect, allowing for the beneficial effects of such a sustained-release form, e.g. the maintenance of even concentrations in the blood.
[1342]Suitable sustained-release forms as well as materials and methods for their preparation are known to those skilled in the art, e.g. from the tables of contents from “Modified-Release Drug Delivery Technology”, Rathbone, M. J. Hadgraft, J. and Roberts, M. S. (Eds.), Marcel Dekker, Inc., New York (2002); “Handbook of Pharmaceutical Controlled Release Technology”, Wise, D. L. (Ed.), Marcel Dekker, Inc. New York, (2000); “Controlled Drug Delivery”, Vol. I, Basic Concepts, Bruck, S. D. (Ed.), CRC Press Inc., Boca Raton (1983) and from Takada, K. and Yoshikawa, H., “Oral Drug delivery”, Encyclopedia of Controlled Drug Delivery, Mathiowitz, E. (Ed.), John Wiley & Sons, Inc., New York (1999), Vol. 2, 728-742; Fix, J., “Oral drug delivery, small intestine and colon”, Encylopedia of Controlled Drug Delivery, Mathiowitz, E. (Ed.), John Wiley & Sons, Inc., New York (1999), Vol. 2, 698-728. The respective descriptions are incorporated by reference and are part of the disclosure.
[1343]If the pharmaceutical formulation according to the present invention comprises at least one of the components (A) and (B) at least partially in a sustained-release form, said sustained release may preferably be achieved by the application of at least one coating or provision of a matrix comprising at least one sustained-release material.
[1344]The sustained-release material is preferably based on an optionally modified, water-insoluble, natural, semisynthetic or synthetic polymer, or a natural, semisynthetic or synthetic wax or fat or fatty alcohol or fatty acid, or on a mixture of at least two of these aforementioned components.
[1345]The water-insoluble polymers used to produce a sustained-release material are preferably based on an acrylic resin, which is preferably selected from the group of poly(meth)acrylates, particularly preferably poly(C1-4)alkyl (meth)acrylates, poly(C1-4)dialkylamino(C1-4)alkyl (meth)acrylates and/or copolymers or mixtures thereof, and very particularly preferably copolymers of ethyl acrylate and methyl methacrylate with a monomer molar ratio of 2:1 (Eudragit NE30F®), copolymers of ethyl acrylate, methyl methacrylate and trimethylammonium ethyl methacrylate-chloride with a monomer molar ratio of 1:2:0.1 (Eudragit RS®), copolymers of ethyl acrylate, methyl methacrylate and trimethylammonium ethyl methacrylate-chloride with a monomer molar ratio of 1:2:0.2 (Eudragit RL®), or a mixture of at least two of the above-mentioned copolymers. These coating materials are commercially available as 30 wt. % aqueous latex dispersions, i.e. as Eudragit RS30D®, Eudragit NE30D® or Eudragit RL30D®, and may also be used as such for coating purposes.
[1346]In another embodiment, the sustained-release material is based on water-insoluble cellulose derivatives, preferably alkyl celluloses, particularly preferably ethyl cellulose, or cellulose esters, e.g. cellulose acetate. Aqueous ethyl cellulose dispersions are commercially available, for example, under the trademarks Aquacoat® or Surelease®.
[1347]As natural, semisynthetic or synthetic waxes, fats or fatty alcohols, the sustained-release material may be based on carnauba wax, beeswax, glycerol monostearate, glycerol monobehenate, glycerol ditripalmitostearate, microcrystalline wax, cetyl alcohol, cetylstearyl alcohol or a mixture of at least two of these components.
[1348]The aforementioned polymers of the sustained-release material may also comprise a conventional, physiologically acceptable plasticizer in amounts known to those skilled in the art.
[1349]Examples of suitable plasticizers are lipophilic diesters of a C6-C40aliphatic or aromatic dicarboxylic acid and a C1-C8aliphatic alcohol, e.g. dibutyl phthalate, diethyl phthalate, dibutyl sebacate or diethyl sebacate, hydrophilic or lipophilic citric acid esters, e.g. triethyl citrate, tributyl citrate, acetyltributyl citrate or acetyltriethyl citrate, polyethylene glycols, propylene glycol, glycerol esters, e.g. triacetin, Myvacet® (acetylated mono- and diglycerides, C23H44O5to C25H42O2), medium-chain triglycerides (Miglyol®), oleic acid or mixtures of at least two of said plasticizers.
[1350]Aqueous dispersions of Eudragit RS® and optionally Eudragit RL® preferably contain triethyl citrate. The sustained-release material may comprise one or more plasticisers in amounts of, for example, 5 to 50 wt. % based on the amount of polymer(s) used.
[1351]The sustained-release material may also contain other conventional auxiliary substances known to those skilled in the art, e.g. lubricants, coloured pigments or surfactants.
[1352]The pharmaceutical formulation of the present invention may also comprise at least one of the components (A) and (B) covered by an enteric coating form which dissolves as a function of pH. Because of this coating, part or all of the pharmaceutical formulation can pass through the stomach undissolved and the components (A) and/or (B) are only released in the intestinal tract. The enteric coating preferably dissolves at a pH of between 5 and 7.5.
[1353]The enteric coating may be based on any enteric material known to those skilled in the art, e.g. on methacrylic acid/methyl methacrylate copolymers with a monomer molar ratio of 1:1 (Eudragit L®), methacrylic acid/methyl methacrylate copolymers with a monomer molar ratio of 1:2 (Eudragit S®), methacrylic acid/ethyl acrylate copolymers with a monomer molar ratio of 1:1 (Eudragit L30D-55®), methacrylic acid/methyl acrylate/methyl methacrylate copolymers with a monomer molar ratio of 7:3:1 (Eudragit FS®), shellac, hydroxypropyl methyl cellulose acetate-succinates, cellulose acetate-phthalates or a mixture of at least two of these components, which can optionally also be used in combination with the above-mentioned water-insoluble poly(meth)acrylates, preferably in combination with Eudragit NE30D® and/or Eudragit RL® and/or Eudragit RS®.
[1354]The coatings of the pharmaceutical formulations of the present invention may be applied by the conventional processes known to those skilled in the art, e.g. from Johnson, J. L., “Pharmaceutical tablet coating”, Coatings Technology Handbook (Second Edition), Satas, D. and Tracton, A. A. (Eds), Marcel Dekker, Inc. New York, (2001), 863-866; Carstensen, T., “Coating Tablets in Advanced Pharmaceutical Solid”, Swarbrick, J. (Ed.), Marcel Dekker, Inc. New York (2001), 455-468; Leopold, C. S., “Coated dosage forms for colon-specific drug delivery”, Pharmaceutical Science & Technology Today, 2(5), 197-204 (1999), Rhodes, C. T. and Porter, S. C., Coatings, in Encyclopedia of Controlled Drug Delivery. Mathiowitz, E. (Ed.), John Wiley & Sons, Inc., New York (1999), Vol. 1, 299-311. The respective descriptions are incorporated by reference and are part of the disclosure.
[1355]In another embodiment, the pharmaceutical formulation of the present invention contains one or both of components (A) and (B) not only in sustained-release form, but also in non-sustained-release form. By combination with the immediately released form, a high initial dose can be achieved for the rapid onset of the beneficial effect. The slow release from the sustained-release form then prevents the beneficial effect from diminishing. Such a pharmaceutical formulation is particularly useful for the treatment of acute health problems.
[1356]This may be achieved, for example, by a pharmaceutical formulation having at least one immediate-release coating comprising at least one of the components (A) and (B) to provide for rapid onset of the beneficial effect after administration to the patient.
[1357]The present invention also relates to the treatment the aforementioned disorders and/or diseases with a combination of at least one compound with 5-HT6receptor affinity and at least one NMDA-receptor ligand which may be administered separately, therefore the invention also relates to combining separate pharmaceutical compositions into a kit form. The kit, according to this invention, comprises two separate pharmaceutical compositions: a first unit dosage form comprising a prophylactically or therapeutically effective amount of at least one NMDA-receptor ligand, or a pharmaceutically acceptable salt or ester thereof, and a pharmaceutically acceptable carrier or diluent in a first unit dosage form, and a second unit dosage form comprising a prophylactically or therapeutically effective amount of at least one compound with 5-HT6receptor affinity, or a pharmaceutically acceptable salt or ester thereof, and a pharmaceutically acceptable carrier or diluent in a second unit dosage form. The kit further comprises a container. Such kits are especially suited for the delivery of solid oral forms such as tablets or capsules. Such a kit preferably includes a number of unit dosages. Such kits can include a card having the dosages oriented in the order of their intended use. An example of such a kit is a “blister pack”. Blister packs are well known in the packaging industry and are widely used for packaging pharmaceutical unit dosage forms. If desired, a memory aid can be provided, for example in the form of numbers, letters, or other markings or with a calendar insert, designating the days or time in the treatment schedule in which the dosages can be administered.
EXAMPLE
Example 1
Test of Novel Object Discrimination in Rats
[1358]Adult male Lister Hooded rats (Charles River, UK) weighing 200-350 g at the start of the experiment were housed in groups of four on a 12:12 h light:dark cycle (lights on at 07:00 h). Food and water were available ad libitum throughout the study, and the room temperature (21±2° C.) and relative humidity (45-65%) were kept constant. In one experiment, rats received i.p. injections (2 ml/kg, −20 minutes prior to the familiarisation trial) of memantine (n=12) at 5, 10, 15 and 20 mg/kg compared with vehicle (0.5% methylcellulose in saline, 2 ml/kg), such that all rats received all doses of the compound in a random order with each behavioural test occurring at seven day intervals.
[1359]In the combination study, a group of rats (n=12 each) received injection of a sub-effective dose of compound 841 (1 mg/kg i.p.) or vehicle (0.5% methylcellulose in saline, 2 ml/kg), either alone or combined with memantine (5 mg/kg). Each drug combination was administered to all rats in the group over a period of 4 weeks in a random order using a seven day behavioural test interval.
[1360]The two trial novel object discrimination paradigm utilised, was as described by Ennanceur and Delacour, (A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31, 47-59, 1988) with minor modification (King et al., “5-ht6 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing consolidation—an effect sensitive to NMDA receptor antagonism”, Neuropharmacol. 47, 195-204, 2004; Woolley et al., “Reversal of a cholinergic-induced deficit in a rodent model of recognition memory by the selective 5-ht6 receptor antagonist, Ro 04-6790”, Psychopharmacol. 170, 358-367, 2003). The twelve open field test arenas used for object discrimination were clear perspex boxes (39×23.5 cm with 24.5 cm high walls) to which each rat was habituated for 60 minutes the day prior to test days. On the test day, for the first experiment, the administration was 20 minutes before acclimatisation. For the combination experiment, the first drug was administered −40 minutes and the second drug −20 minutes before the familiarisation trial. Therefore, 20 minutes after the injection each rat received 3 minutes acclimatisation to the perspex box in absence of objects which was then followed by the 3 minute familiarisation trial and a second 3 minute choice trial following a 4 hour inter-trial interval. During both trials, exploration of each object was defined as the time spent (s) sniffing (within 1 cm of it with active vibrissae), licking, chewing or touching the object with the nose.
[1361]The results obtained for the first trial were shown in FIG. 1. As can be seen, when memantine is administered alone, high amounts of said compound are necessary for rats to discriminate a novel object against a familiar object. On the contrary, as shown in FIG. 2 for the second trial, the combined administration of memantine and compound 841 in lower doses (5 and 1 mg/kg, respectively) makes the animal to spend more time exploring the novel/unknown object than when the object is familiar for him, thus proving a better ability to memorize or distinguish a novel from a known object.