Инвестиции
Стартапам
Инвесторам
Инвесторам
Аналитика
Инвестиции
Стартапам
Инвесторам
Аналитика
Спрос и кооперация
Спрос и кооперация
Развитие и продвижение бизнеса
Спрос
Кооперация
Спрос и кооперация
Развитие и продвижение бизнеса
Спрос
Кооперация
Гранты и кредиты
Гранты и кредиты
Инфраструктура
Инфраструктура
Современные пространства
для бизнеса
для бизнеса
Инфраструктура
Современные пространства
для бизнеса
для бизнеса
Патентование
Патентование
Обучение и карьера
Обучение и карьера
Проекты для мегаполиса
Проекты для мегаполиса
Технологические городские проекты
Проекты для мегаполиса
О Кластере
О Кластере
Лидеры цифровой трансформации
Лидеры цифровой трансформации
Академия инноваторов
Академия инноваторов
Новатор Москвы
Новатор Москвы
Обучение и карьера
Обучение и карьера
Образовательные программы и база знаний
Обучение
Обучение и карьера
Образовательные программы и база знаний


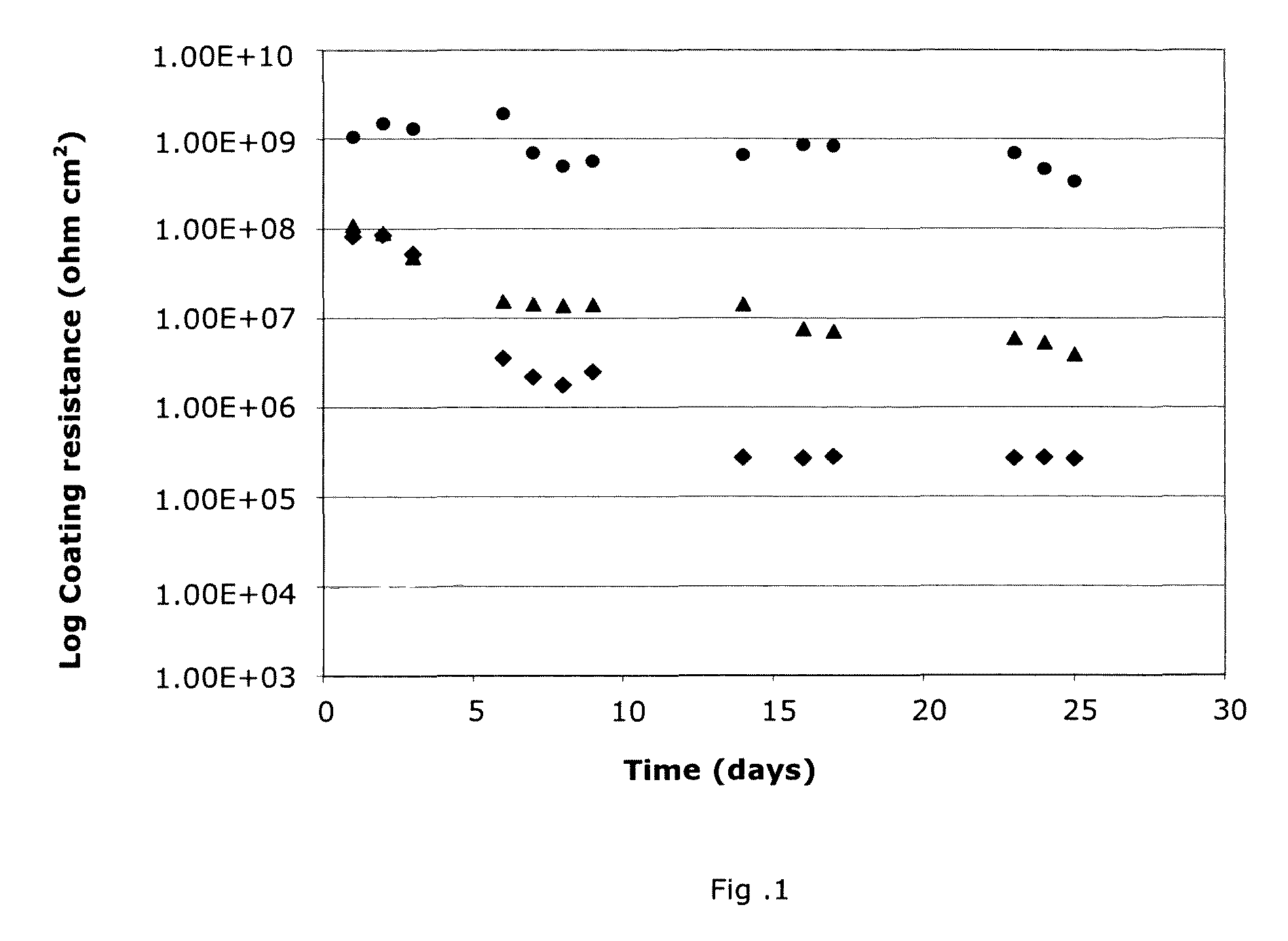
 Accelerated corrosion test
Accelerated corrosion test 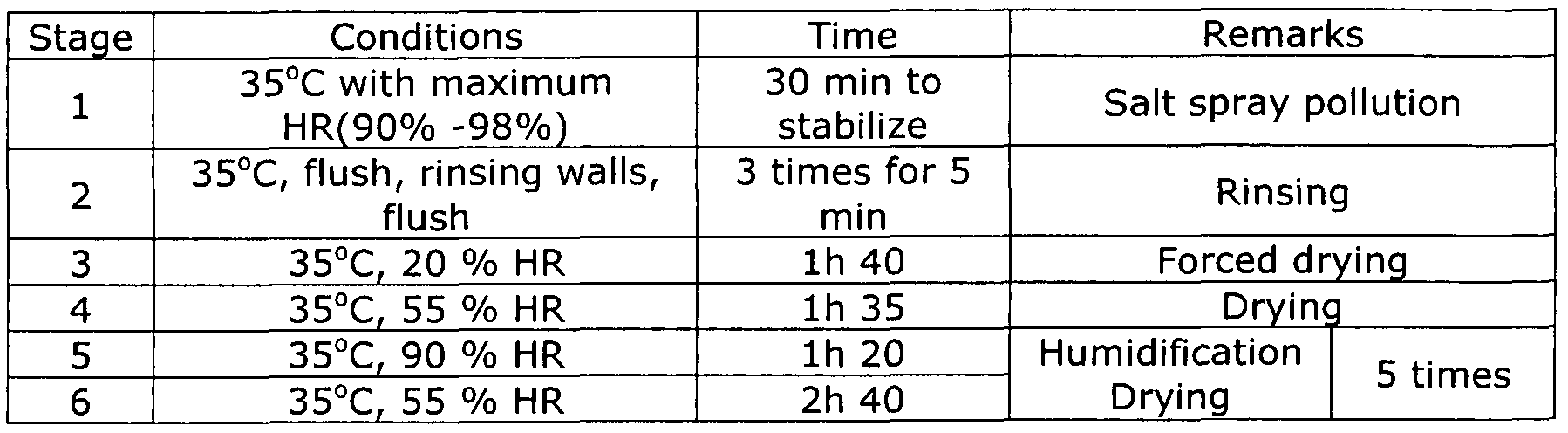 The test was performed in a WEISS type SC/KKT-1000 cabinet. The cabinet uses a solution of l%NaCI at pH 4 (acidified with H2S04) and a conductivity of <5 μ≤ (micro-Siemens). The results obtained after 10 weeks of the ECCl test are given in Table 4.
The test was performed in a WEISS type SC/KKT-1000 cabinet. The cabinet uses a solution of l%NaCI at pH 4 (acidified with H2S04) and a conductivity of <5 μ≤ (micro-Siemens). The results obtained after 10 weeks of the ECCl test are given in Table 4. 

