[0001]The present invention relates to a composition for artificially colouring the skin, comprising, in a physiologically acceptable medium:
- a) at least one self-tanning agent, and
- b) at least one dye that can be obtained by reacting dehydroascorbic acid and/or a monomeric derivative thereof of formula (I) and/or an isomer thereof of formula (I′) which will be defined hereinbelow and/or a polymeric derivative thereof, especially dimers of formula (II) which will be defined hereinbelow, with a compound comprising at least one free amine function.
[0004]The present invention also relates to a process for artificially colouring the skin, comprising the application of a composition comprising, in a physiologically acceptable medium:
- a) at least one self-tanning agent, and
- b) at least one dye that can be obtained by reacting dehydroascorbic acid and/or a monomeric derivative thereof of formula (I) and/or an isomer thereof of formula (I′) which will be defined hereinbelow and/or a polymeric derivative thereof, especially dimers of formula (II) which will be defined hereinbelow, with a compound comprising at least one free amine function.
[0007]It is common for people with pigmentation marks, or shadows under the eyes, to wish to correct these cutaneous dyschromias and to use for this purpose cosmetic or dermatological compositions that can make the complexion uniform. To this end, it is known practice to use covering products for the purpose of making the complexion immediately uniform, which products do indeed hide skin imperfections, but have the major drawback of masking the natural appearance of the skin (mask sensation).
[0008]It is also known practice to use products containing interference pigments such as nacres, which, although being able to hide skin imperfections, have the major drawback of giving the skin a shiny, unnatural appearance.
[0009]Finally, it is known practice to use self-tanning products based on carbonyl derivatives such as dihydroxyacetone (DHA), which allow, via interaction with the free amine functions of the skin, in particular the amino acids, peptides and proteins of the skin, the formation of coloured products. These products, especially those based on DHA, by giving a tanned, healthy-complexion appearance, allow skin imperfections to be hidden.
[0010]One drawback of DHA is the length of time the coloration takes to develop: specifically, several hours (3 to 5 hours in general) are required for the coloration to be revealed. Another drawback of DHA is its tendency to produce yellow shades that harm the production of a natural skin tone. There is thus increasing demand for self-tanning products that act quickly and give a coloration closer to that of a natural tan.
[0011]In order to overcome the problem of kinetics and to allow visualization of the application of the product (for better homogeneity of the results), it is possible to combine DHA with dyes. However, the susceptibility of DHA with respect to numerous chemical species limits the possible combinations (e.g.: coloured iron oxides, and water-soluble azo, quinone or xanthene dyes).
[0012]Thus, the search continues for a novel process of artificial non-covering coloration of the skin that can rapidly give the skin a uniform complexion to correct cutaneous dyschromias, without the drawbacks mentioned previously and without hiding the natural appearance of the skin.
[0013]Patent application WO 2005/039 510 discloses the use of dehydroascorbic acid or a salt thereof produced in situ via enzymatic oxidation, as a fixing agent in the permanent waving of hair. Patent application DE 197 45 354 also discloses the use of dehydroascorbic acid in combination with particular compounds with primary or secondary amine groups or hydroxyl groups, for colouring the hair.
[0014]After extensive studies conducted in the field of artificial colouring of the skin, the Applicant has now discovered that by combining a self-tanning agent with a dye that can be obtained by reacting dehydroascorbic acid or a monomer or polymer thereof with a compound containing a free amine function, it is possible to obtain an immediate healthy-complexion effect which is then reinforced over time. The compositions according to the invention are non-covering and do not give the skin a shiny, unnatural appearance.
[0015]The present invention thus relates to a composition for artificially colouring the skin, comprising, in a physiologically acceptable medium:
- a) at least one self-tanning agent, and
- b) at least one dye that can be obtained by reacting dehydroascorbic acid and/or a monomeric derivative thereof of formula (I) and/or an isomer thereof of formula (I′) which will be defined hereinbelow and/or a polymeric derivative thereof, especially dimers of formula (II) which will be defined hereinbelow, with a compound comprising at least one free amine function.
[0018]The present invention also relates to a process for artificially colouring the skin, comprising the application of a composition comprising, in a physiologically acceptable medium:
- a) at least one self-tanning agent, and
- b) at least one dye that can be obtained by reacting dehydroascorbic acid and/or a monomeric derivative thereof of formula (I) and/or an isomer thereof of formula (I′) which will be defined hereinbelow and/or a polymeric derivative thereof, especially dimers of formula (II) which will be defined hereinbelow, with a compound comprising at least one free amine function.
[0021]For the purposes of the present invention, the expression “artificial coloration of the skin” means a long-lasting, non-covering coloration (i.e. a coloration that does not have a tendency to opacify the skin), which is not removed either with water or using a solvent, and which is resistant both to rubbing and to washing with a solution containing surfactants. Such a long-lasting coloration is thus distinguished from the superficial and temporary coloration provided, for example, by a makeup product.
[0022]For the purposes of the present invention, the term “polymer” will mean any molecule having in its structure at least two repeating units.
[0023]For the purposes of the present invention, the expression “physiologically acceptable medium” means a support that is compatible with the skin, the nails, the lips, the eyelashes and the eyebrows, which has a pleasant colour, odour and feel and which does not give rise to any unacceptable discomfort (stinging, tautness or redness) liable to put the consumer off using this composition comprising such a support.
[0024]Other characteristics, aspects and advantages of the present invention will emerge on reading the detailed description that follows.
[0025]The self-tanning agents are generally chosen from certain monocarbonyl or polycarbonyl compounds, for instance isatin, alloxan, ninhydrin, glyceraldehyde, mesotartaric aldehyde, glutaraldehyde, erythrulose, pyrazoline-4,5-dione derivatives as described in patent application FR 2 466 492 and WO 97/35842, dihydroxyacetone (DHA), and 4,4-dihydroxypyrazolin-5-one derivatives as described in patent application EP 903 342. DHA will preferably be used.
[0026]DHA may be used in free form and/or encapsulated, for example in lipid vesicles such as liposomes, described especially in patent application WO 97/25970.
[0027]The self-tanning agent(s) is (are) generally present in proportions ranging from 0.1% to 15% by weight, preferably from 0.2% to 10% by weight and more preferentially from 1% to 8% by weight relative to the total weight of the composition.
[0028]The dye used in accordance with the present invention may be obtained by reacting dehydroascorbic acid or a monomeric derivative thereof of formula (I) or an isomeric form thereof of formula (I′) or a polymeric derivative thereof with an amino acid.
[0029]Dehydroascorbic acid and the monomeric derivatives in accordance with the invention correspond to formula (I) below or correspond to the isomeric form thereof of formula (I′) below:
[0000]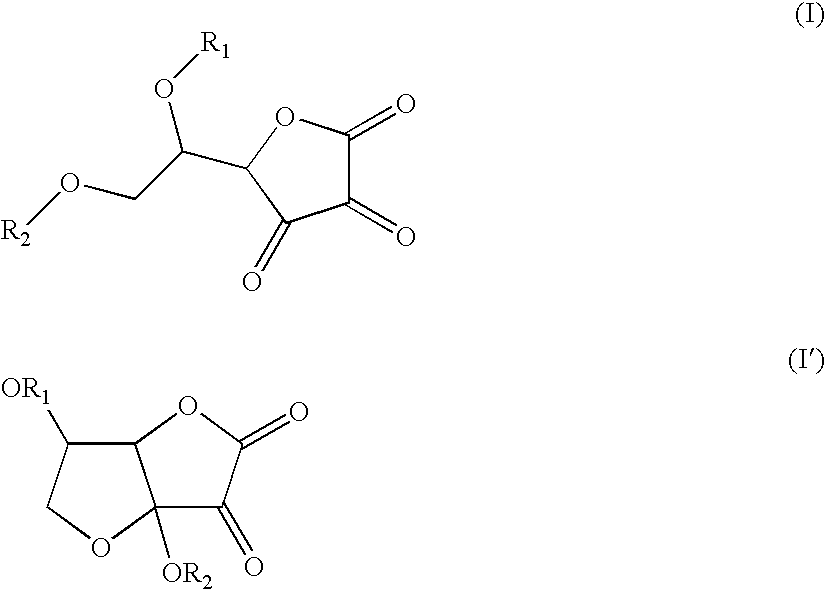
[0000]in which OR1 and OR2, which may be identical or different, denote OH; a linear or branched, saturated or unsaturated C1-C30 and more preferentially C1-C18 alkoxy radical; a glycoside and more preferentially glucose; a linear or branched, saturated or unsaturated C1-C30 (preferably C1-C18) aliphatic carboxylic acid ester, which may be substituted with an aryl group or a heterocycle; an aryl or heterocyclic carboxylic acid ester which may be substituted with at least one linear or branched, saturated or unsaturated C1-C30 (more preferentially C1-C18) alkyl radical; a phosphate group; a sulfate group.
[0030]Preferentially, R2 will denote a linear or branched, saturated or unsaturated C1-C30 (preferably C1-C18) aliphatic carboxylic acid ester, which may be substituted with an aryl group or a heterocycle; an aryl or heterocyclic carboxylic acid ester which may be substituted with at least one linear or branched, saturated or unsaturated C1-C30 (more preferentially C1-C18) alkyl radical.
[0031]Dehydroascorbic acid of formula (I) is also known as threo-2,3-hexodiulosono-1,4-lactone, 9CI (CAS # 490-83-5) and has the structure:
[0000]
[0032]The isomeric form thereof of formula (I′) known as 3a,6-dihydroxy-tetrahydro-furo[3,2-b]furan-2,3-dione has the structure:
[0000]
[0033]Among the monomeric derivatives of dehydroascorbic acid of formula (I) and (II) that may be mentioned in particular are the following particular compounds:
[0034]L-threo-2,3-hexodiulosonic acid, 7-lactone, 5,6-bis(3-phenyl-2-propenoate)
[0000](CAS # 106406-96-6) with OR1=OR2=3-phenyl-2-propenoate
[0035]L-threo-2,3-hexodiulosonic acid, γ-lactone, 6-acetate (CAS # 106227-O2-5) with OR2=acetate and R1=H
[0036]L-threo-2,3-hexodiulosonic acid, γ-lactone, 5,6-diacetate
[0000](CAS # 59681-41-3) with OR1=OR2=acetate
[0037]L-threo-2,3-hexodiulosonic acid, γ-lactone, 6-hexadecanoate
[0000](CAS # 63247-05-2) with OR2=hexadecanoate and OR1=H
[0038]L-threo-2,3-hexodiulosonic acid, γ-lactone, 6-octadecanoate (9CI)
[0000](CAS # 59681-40-2) with OR2=octadecanoate and OR1=OH
[0039]L-threo-2,3-hexodiulosonic acid, γ-lactone, 6-benzoate
[0000](CAS # 63247-04-1) with OR2=benzoate and OR1=OH.
[0040]Among the examples of polymeric derivatives that may be mentioned are the dimeric compounds of formula (II) below:
[0000]
[0000]in which OR1, OR2, OR3 and OR4, which may be identical or different, denote OH; a linear or branched, saturated or unsaturated C1-C30 and more preferentially C1-C18 alkoxy radical; a glycoside and more preferentially glucose; a linear or branched, saturated or unsaturated C1-C30 (preferably C1-C18) aliphatic carboxylic acid ester, which may be substituted with an aryl group or a heterocycle; an aryl or heterocyclic carboxylic acid ester which may be substituted with at least one linear or branched, saturated or unsaturated C1-C30 (more preferentially C1-C18) alkyl radical; a phosphate group; a sulfate group.
[0041]Preferentially, R1 and/or R2 will denote a linear or branched, saturated or unsaturated C1-C30 (preferably C1-C18) aliphatic carboxylic acid ester, which may be substituted with an aryl group or a heterocycle; an aryl or heterocyclic carboxylic acid ester which may be substituted with at least one linear or branched, saturated or unsaturated C1-C30 (more preferentially C1-C18) alkyl radical.
[0042]Among the dehydroascorbic acid-based dimers of formula (II), mention may be made in particular of the following particular compounds:
- 2H,8H-bisfuro[3′,2′:2,3]furo[3,4-b:3′,4′-e][1,4]dioxin-5,11(5aH,11aH)-dione,
- 3,5a,9,11a-tetrakis(benzoyloxy)tetrahydro-[3S-(3α,3aα,5aβ,6aS*,9aα,11aβ,12aS*)] (CAS # 103559-39-3) with OR1=OR2=OR3=OR4=benzoate
- 1,6,9,13-tetraoxadispiro[4.2.4.2]tetradecane-7,14-dicarboxylic acid, 4,12-dihydroxy-3,7,11,14-tetramethoxy-, di-γ-lactone
(CAS # 94329-25-6) with R1=R2=R3=R4=methyl
- 2H,8H-bisfuro[3′,2′:2,3]furo[3,4-b:3′,4′-e][1,4]dioxin-5,11(5aH,11aH)-dione,
- 3,5a,9,11a-tetrakis(acetyloxy)tetrahydro-[3S-(3α,3aα,5aβ,6aS*,9α,9aα,11aβ,12aS*)] (CAS # 25726-18-5) with OR1═OR2═OR3═OR4=acetate
- 2H,8H-bisfuro[3′,2′:2,3]furo[3,4-b:3′,4′-e][1,4]dioxin-5,11(5aH,11aH)-dione,
- 3,5a,9,11a-tetrakis(3″-phenyl-2″-propenoxy)-tetrahydro-[3S-(3α,3aα,5aβ,6aS*,9α,9aα,11aβ,12aS*)] (CAS # 106406-97-7) with OR1═OR2═OR3═OR4=3-phenyl-2-propenoate.
[0050]Mention will be made more particularly of the dimeric compound (CAS # 72691-25-29) with R1═R2=H, having the following structure:
[0000]
[0051]The compounds containing a free amine function in accordance with the invention are preferably chosen from amino acids, proteins, oligopeptides or polypeptides, or protein hydrolysates, or mixtures thereof.
[0052]The compounds containing a free amine function in accordance with the invention are preferably chosen from amino acids, proteins, oligopeptides or polypeptides, or protein hydrolysates, or mixtures thereof.
[0053]Among the amino acids that may be used according to the invention, examples that may be mentioned include α-amino acids obtained by hydrolysis of animal or plant proteins, for instance collagen, keratin, casein, elastin, soybean protein and wheat or almond glutens. Mention may also be made of arginine, glycine, tyrosine, alanine, phenylalanine, dihydroxyphenyl-alanine (DOPA), ornithine, lysine, polylysines especially with a molecular weight ranging from 100 000 to 80 000 daltons, spermitine, citrulline, tryptophan, 6-aminocapronic acid, isoleucine, leucine, methionine, serine, proline, valine, hydroxyproline, aspartic acid, tyrosine, taurine, Val-Tyr-Val, L-asparagine, Lys-Lys-Lys-Lys and Cys-Gly.
[0054]Among the peptides that may be mentioned are glutathione (GSH), and collagen, keratin, casein, elastin, soybean protein, wheat gluten or almond protein hydrolysates.
[0055]Valine, glutamine, glutathione, L-phenylalanine, serine, glycine, Val-Tyr-Val, alanine or methionine will particularly be used, and more particularly glycine.
[0056]The dehydroascorbic acid-based dye according to the invention may be obtained according to a preparation process comprising the following steps:
[0000](i) dehydroascorbic acid or the monomeric derivative or polymeric derivative thereof is diluted in a water/C1-C4 alcohol (preferably ethanol) mixture,
(ii) the compound containing a free amine function is added to the solution obtained,
(iii) the reaction mixture is heated to a temperature ranging from 80 to 100° C. until the desired shade is obtained,
(iv) the reaction is stopped by cooling to room temperature,
(v) the final dye is extracted by evaporation or by filtration.
[0057]The dye derived from dehydroascorbic acid or a monomeric or polymeric derivative thereof is preferably present in the compositions of the invention in contents ranging from 0.1% to 25% by weight, more preferentially from 0.5% to 15% by weight and more particularly from 0.5% to 10% by weight relative to the total weight of the composition containing it.
[0058]The dehydroascorbic acid or one of the monomeric, polymeric or isomeric derivatives thereof may be obtained from ascorbic acid or a derivative thereof or a salt thereof via oxidation with a chemical oxidizing agent and/or an enzymatic oxidizing agent according to the following reaction scheme:
[0000]
[0059]The oxidation reaction may in particular be efficiently catalysed by many types of enzymes, for instance the ascorbate oxidases produced by the majority of plants and also certain bacteria, yeasts or animals (E.C [1.10.3.3] Lee, M. H.; Dawson, C. R. Methods Enzymol., 1979, 62, 30-39).
[0060]The chemical oxidizing agents conventionally used for the oxidation of ascorbic acid or derivatives or salts thereof are, for example, hydrogen peroxide, urea peroxide, alkali metal bromates, persalts such as perborates and persulfates, and peracids, or mixtures thereof.
[0061]The enzymatic oxidizing systems used are conventionally oxidase enzymes using either atmospheric oxygen or a substrate to form hydrogen peroxide, among which mention may be made of 2-electron oxidoreductases such as uricases, ascorbate oxidases, etc.
[0062]The enzymes used in accordance with the present invention are preferably chosen from the ascorbate oxidases using atmospheric oxygen. More preferentially, the enzymes are chosen from those belonging to the Enzyme Commission classification [1.10.3.3].
[0063]The ascorbate oxidase enzyme may be derived, for example, from the following botanic genera: Arabidopsis, Brassica, Cucumis, Curcubita, Myrothecium, Nicotiana, Oryza, Triticum. It is more preferentially chosen from those derived from Curbita pepo mudullosa (aubergine). It is also possible to use an ascorbate oxidase enzyme obtained from numerous other plants, including cabbage (Brassica oleracea), cucumber (Cucumis savitus), pumpkin (Curcubita cv. Ebisu Nankin), tobacco (Nicotiana tabacum), mustard (Sinapsis alba), rice (Oryza sativa) and wheat (Triticum aestivum). Other sources include mushrooms (Myrotectium verrucaria) and thermophilic bacteria (Acremonium sp. HI-25).
[0064]The enzyme may be present as a solution or in powder form and may preferably be stabilized with buffers, glycerol, sugars or other polyhydroxylated compounds, metal-chelating agents such as EDTA, thiols such as thioglycerol, mercaptoethanol or dithiothreitol, polyethylene glycol, unreactive proteins or other enzyme-preserving agents. The enzymes may also be stabilized via covalent modification according to standard techniques. The enzyme may also be immobilize by covalent bonding onto a solid support such as surface-modified silica, alumina, glass, oxirane-modified polymethacrylate, carboxyalkylcellulose, aminoalkyl silica, aminoalkyl glass or aminoalkylcellulose microparticles. The enzymes may also be adsorbed onto the surfaces of hydrophobically or ionically modified particles such as carboxyalkylcelluloses or dialkylaminocelluloses.
[0065]Another possibility consists in covalently bonding the enzyme with a synthetic or biosynthetic water-soluble polymer, such as polyethylene glycols, poly(acrylic acids), poly(vinyl alcohols), polyethyleneimines, dextran and proteins such as gelatin or uricase.
[0066]The said enzyme is preferably present in the composition resulting from the mixing of components (B) and (C) in contents ranging from 1 to 10 000 ppm and preferably 100 to 1000 ppm.
Galenical Forms
[0067]The compositions of the invention may be in any form that is suitable for topical application, especially in the form of aqueous gels, in the form of emulsions obtained by dispersing a fatty phase (also known as an oily phase) in an aqueous phase (O/W) or, conversely, (W/O) or multiple emulsions (for example W/O/W, O/W/O or O/O/W). They may be more or less fluid and may have the appearance of a white or coloured cream, a pomade, a milk, a lotion, a serum, a paste, a powder or a solid tube, and they may optionally be packaged as an aerosol and be in the form of a mousse or spray. These compositions are prepared according to the usual methods.
[0068]According to one particular embodiment of the invention, the compositions of the invention may be in the form of an emulsion and in this case may comprise at least one oily phase. The proportion of the oily phase of the emulsion may range from 1% to 80% by weight, preferably from 2% to 50% by weight and better still from 2% to 40% by weight relative to the total weight of the composition. The fatty substances of the oily phase, especially the oils, and the emulsifiers and coemulsifiers that may be present, used in the composition in emulsion form are chosen from those conventionally used in cosmetics or dermatology. The emulsifier and the coemulsifier, when they are present, are generally in a proportion ranging from 0.1% to 30% by weight, preferably from 0.3% to 20% by weight and better still from 0.5% to 15% by weight relative to the total weight of the composition. The emulsion may also contain lipid vesicles in addition to or instead of the emulsifiers and/or coemulsifiers.
[0069]The emulsions generally contain at least one emulsifier chosen from amphoteric, anionic, cationic and nonionic emulsifiers, used alone or as a mixture. The emulsifiers are chosen in an appropriate manner according to the continuous phase of the emulsion to be obtained (W/O or O/W). When the emulsion is multiple, it generally comprises an emulsifier in the primary emulsion and an emulsifier in the outer phase into which the primary emulsion is introduced.
[0070]As emulsifiers that may be used for the preparation of the W/O emulsions, examples that may be mentioned include alkyl esters or ethers of sorbitan, of glycerol or of sugars; silicone surfactants, for instance dimethicone copolyols such as the mixture of cyclomethicone and of dimethicone copolyol, sold under the names DC 5225 C and DC 3225 C by the company Dow Corning, and alkyldimethicone copolyols such as laurylmethicone copolyol sold under the name “Dow Corning 5200 Formulation Aid” by the company Dow Corning, cetyl dimethicone copolyol sold under the name Abil EM 90® by the company Goldschmidt, and the mixture of polyglyceryl-4 isostearate/cetyl dimethicone copolyol/hexyl laurate sold under the name Abil WE 09® by the company Goldschmidt. One or more co-emulsifiers may also be added thereto, which may be chosen, advantageously, from the group comprising branched-chain fatty acid esters of polyol, and especially branched-chain fatty acid esters of glycerol and/or of sorbitan, for example polyglyceryl isostearate, such as the product sold under the name Isolan GI 34 by the company Goldschmidt, sorbitan isostearate, such as the product sold under the name Arlacel 987 by the company ICI, sorbitan glyceryl isostearate, such as the product sold under the name Arlacel 986 by the company ICI, and mixtures thereof.
[0071]As emulsifiers that may be used for the preparation of the O/W emulsions, examples that may be mentioned include nonionic emulsifiers such as fatty acid esters of oxyalkylenated (more particularly polyoxyethylen-ated) polyols, for example polyethylene glycol stearates, for instance PEG-100 stearate, PEG-50 stearate and PEG-40 stearate; fatty acid esters of oxyalkylenated sorbitan comprising, for example, from 20 to 100 EO, for example those sold under the trade names Tween 20 or Tween 60 by the company Uniqema; oxyalkylenated (oxyethylenated and/or oxypropylenated) fatty alkyl ethers; alkoxylated or non-alkoxylated sugar esters, for instance sucrose stearate such as PEG-20 methylglucose sesquistearate; sorbitan esters such as the sorbitan palmitate sold under the name Span 40 by the company Uniqema; diacid esters of fatty alcohols, for instance dimyristyl tartrate; mixtures of these emulsifiers, for instance the mixture of glyceryl stearate and of PEG-100 stearate (CTFA name: Glyceryl Stearate/PEG-100 Stearate) sold under the name Arlacel 165 by the company Uniqema and under the name Simulsol 165 by the company SEPPIC; or the mixture of dimyristyl tartrate, cetearyl alcohol, Pareth-7 and PEG-25 laureth-25, sold under the name Cosmacol PSE by the company Sasol (CTFA name: Dimyristyl tartrate/cetearyl alcohol/12-15 Pareth 7/PPG 25 laureth 25).
[0072]Coemulsifiers such as, for example, fatty alcohols containing from 8 to 26 carbon atoms, for instance cetyl alcohol, stearyl alcohol and the mixture thereof (cetearyl alcohol), octyldodecanol, 2-butyloctanol, 2-hexyldecanol, 2-undecylpentadecanol or oleyl alcohol, or fatty acids, may be added to these emulsifiers.
[0073]It is also possible to prepare emulsions without emulsifying surfactants or containing less than 0.5% of them relative to the total weight of composition (A) or (B), by using suitable compounds, for stabilizing the said emulsions, for example amphiphilic polymers or electrolytes.
Additives
[0074]When the composition of the invention is in emulsion form, it comprises at least one oily phase that contains at least one oil, especially a cosmetic oil. The term “oil” means a fatty substance that is liquid at room temperature (25° C.).
[0075]As oils that may be used in the composition of the invention, it is possible to use, for example, hydrocarbon-based oils of animal origin, such as perhydrosqualene (or squalane); hydrocarbon-based oils of plant origin, such as caprylic/capric acid triglycerides, for instance those sold by the company Stearineries Dubois or those sold under the names Miglyol 810, 812 and 818 by the company Dynamit Nobel, or alternatively oils of plant origin, for instance sunflower oil, corn oil, soybean oil, marrow oil, grapeseed oil, sesame seed oil, hazelnut oil, apricot oil, macadamia oil, arara oil, coriander oil, castor oil, avocado oil, jojoba oil and shea butter oil; synthetic oils; silicone oils, for instance volatile or non-volatile polymethylsiloxanes (PDMSs) containing a linear or cyclic silicone chain, which are liquid or pasty at room temperature; fluoro oils, such as partially hydrocarbon-based and/or silicone-based fluoro oils, for instance those described in document JP-A-2 295 912; ethers, such as dicaprylyl ether (CTFA name: Dicaprylyl ether); C12-C15 fatty alkyl benzoates (Finsolv TN from Finetex); arylalkyl benzoate derivatives, for instance 2-phenylethyl benzoate (X-Tend 226 from ISP); and amido oils, for instance isopropyl N-lauroylsarcosinate (Eldew SL-205 from Ajinomoto); and mixtures thereof.
[0076]The composition of the invention may also contain one or more organic solvents that may be chosen from the group consisting of hydrophilic organic solvents, lipophilic organic solvents and amphiphilic solvents, or mixtures thereof.
[0077]Examples of hydrophilic organic solvents that may be mentioned include linear or branched monohydric alcohols containing from 1 to 8 carbon atoms, for instance ethanol, propanol, butanol, isopropanol or isobutanol; polyethylene glycols containing from 6 to 80 ethylene oxides; polyols such as propylene glycol, isoprene glycol, butylene glycol, glycerol or sorbitol; monoalkyl or dialkyl isosorbides in which the alkyl groups contain from 1 to 5 carbon atoms, for instance dimethyl isosorbide; glycol ethers, for instance diethylene glycol monomethyl ether or monoethyl ether and propylene glycol ethers, for instance dipropylene glycol methyl ether.
[0078]Amphiphilic organic solvents that may be mentioned include polypropylene glycol (PPG) derivatives such as fatty acid esters of polypropylene glycol, and derivatives of PPG and of fatty alcohols, for instance PPG-23 oleyl ether, and PPG-36 oleate.
[0079]Examples of lipophilic organic solvents that may be mentioned include fatty esters such as diisopropyl adipate, dioctyl adipate or alkyl benzoates.
[0080]The compositions in accordance with the present invention may also comprise standard cosmetic adjuvants chosen from softeners, humectants, opacifiers, stabilizers, emollients, silicones, antifoams, fragrances, preserving agents, anionic, cationic, nonionic, zwitterionic or amphoteric surfactants, fillers, polymers, propellants, and acidifying or basifying agents, or any other ingredient usually used in cosmetics and/or dermatology.
[0081]Hydrophilic thickeners that may be mentioned include carboxyvinyl polymers such as carbopols (carbomers) and the Pemulen products (acrylate/C10-C30-alkylacrylate copolymer) and homopolymers and copolymers of acrylamide and/or of 2-acrylamido-2-methylpropane-sulfonic acid (AMPS), for instance sodium polyacryloyl-dimethyltaurate (and) polysorbate 80 (and) sorbitan oleate sold under the trade name Simulgel 800 by the company SEPPIC; cellulose derivatives such as hydroxyethylcellulose; polysaccharides and especially gums such as xanthan gum; and mixtures thereof.
[0082]Lipophilic thickeners that may be mentioned include modified clays such as hectorite and derivatives thereof, for instance the products sold under the name Bentone.
[0083]Preserving agents that may be mentioned include para-hydroxybenzoic acid esters, also known as Parabens® (in particular methyl paraben, ethyl paraben and propyl paraben), phenoxyethanol, formaldehyde generators, for instance imidazolidinylurea or diazolidinylurea, chlorhexidine digluconate, sodium benzoate, caprylyl glycol, iodopropynyl butyl carbamate, pentylene glycol, alkyltrimethylammonium bromides such as myristyl-trimethylammonium bromide (CTFA name: myrtrimonium bromide), dodecyltrimethylammonium bromide, hexadecyl-trimethylammonium bromide, and mixtures thereof such as the mixture sold under the name Cetrimide® by the company FEF Chemicals. The preserving agent may be present in the composition according to the invention in a content ranging from 0.001% to 10% by weight, especially ranging from 0.1% to 5% by weight and in particular ranging from 0.2% to 3% by weight relative to the total weight of the composition.
[0084]According to one particular form of the invention, in order to improve the stability of the dehydroascorbic acid or a polymer thereof and/or that of ascorbic acid or a salt or derivative thereof, each of these active agents may be encapsulated according to standard encapsulation techniques.
Stabilizers
[0085]In order to improve the stability of the compositions in accordance with the invention, the said composition may also include one or more stabilizers.
[0086]Examples of stabilizers that may be mentioned include
[0088](2) chelating agents,
[0089](3) non-crosslinked N-vinylimidazole polymers or copolymers such as those described in patent application EP 1 316 302.
[0090]According to the invention, the expression “non-crosslinked N-vinylimidazole polymer or copolymer” means any polymer comprising N-vinylimidazole units, and not comprising a crosslinking agent. Copolymers that are suitable for use in the invention are, for example, copolymers comprising N-vinylimidazole units and N-vinylpyrrolidone and/or N-vinylcaprolactam units.
[0091]In one advantageous aspect of the invention, the copolymer has a mole fraction of N-vinylimidazole units of between 0.1 and 1 and more preferentially between 0.4 and 0.9.
[0092]According to one advantageous aspect of the invention, the mole ratio between the N-vinylimidazole unit equivalent and the oxidation-sensitive hydrophilic active agent ranges between 0.004 and 16 and preferentially between 0.01 and 1.
[0093]An N-vinylimidazole/N-vinylpyrrolidone copolymer will preferentially be used.
[0094]The weight-average molar mass of the N-vinylimidazole polymers will advantageously be between 1000 and 1×107 and preferably between 5000 and 5×106.
[0095]The vinylpyrrolidone/vinylimidazole (50/50) copolymer with a weight-average molar mass of 1 200 000 sold under the reference Luvitec VPI 55K72W by the company BASF or the vinylpyrrolidone/vinylimidazole (50/50) copolymer with a weight-average molar mass of 10 000 sold under the reference Luvitec VPI 55K18P by the company BASF may be used for this purpose. The polymers or copolymers according to the invention may be prepared, for example, according to the method described in patent application WO 97/45517.
[0096](4) Amphiphilic polymers chosen from polyisobutylene-based oligomers or polymers comprising a polyisobutylene apolar portion containing at least 40 carbon atoms and at least one polar end portion constituted of carboxylic or dicarboxylic acids, anhydrides thereof or modified forms thereof in the form of esters, amides or salts, and mixtures thereof as described in patent application 1 481 677, can also be used as stabilizers.
[0097]These amphiphilic polymers are constituted of a polyisobutylene apolar portion and of at least one polar portion.
[0098]The polyisobutylene apolar portion contains at least 40 carbon atoms and preferably from 60 to 700 carbon atoms. It is important for this portion to contain at least 40 carbon atoms in order to achieve the aim of the invention. If there are less than 40 carbon atoms, a satisfactorily stable system is not obtained.
[0099]The polar portion of these amphiphilic polymers or oligomers is constituted of carboxylic or dicarboxylic acids, anhydrides thereof or modified forms thereof in the form of esters, amides or salts, and mixtures thereof. Preferably, the polar end portion is constituted of dicarboxylic acids or anhydrides thereof or of modified forms thereof in the form of esters, amides or salts.
[0100]The expression “modified forms in the form of esters, amides or salts” denotes carboxylic or dicarboxylic acids modified with alcohols, amines, alkanolamines or polyols, or alternatively in the form of alkali metal, alkaline-earth metal or ammonium salts or alternatively in the form of salts of an organic base, for instance the diethanolamine and triethanolamine salts.
[0101]The oligomers or polymers derived from succinic acid or anhydride may be chosen especially from the polyisobutylene derivatives of succinic acid or anhydride described in U.S. Pat. No. 4,234,435, U.S. Pat. No. 4,708,753, U.S. Pat. No. 5,129,972, U.S. Pat. No. 4,931,110, GB-A-2 156 799 and U.S. Pat. No. 4,919,179. The polyisobutylene portion may be hydrogenated or non-hydrogenated, with a molecular weight ranging from 400 to 5000. In the succinic-terminated polyisobutylene thus obtained, the succinic portion may be esterified, amidated or in salt form, i.e. it may be advantageously modified with alcohols, amines, alkanolamines or polyols, or alternatively may be in the form of alkali metal, alkaline-earth metal or ammonium salts or alternatively in the form of a salt of an organic base, for instance the diethanolamine and triethanolamine salts. The esterified or amidated succinic-terminated polyisobutylenes are products of reaction of (a) a polyisobutylene containing succinic end groups, and (b) an amine or an alcohol, to form an amide or an ester. The term “amine” used herein includes all types of amines, including alkanolamines. They may be, for example, primary, secondary or tertiary monoamines, these amines possibly being aliphatic, cycloaliphatic, aromatic or heterocyclic, and saturated or unsaturated. Moreover, the alcohols may be monoalcohols or polyalcohols. The monoalcohols comprise primary, secondary or tertiary aliphatic alcohols, and phenols. The polyalcohols may be chosen, for example, from aliphatic, cycloaliphatic, aromatic and heterocyclic polyalcohols. The modified (esterified or amidated) succinic-terminated polyisobutylenes and the process for preparing them are described in particular in document U.S. Pat. No. 4,708,753.
[0102]Succinic-terminated polyisobutylenes that may especially be mentioned include modified succinic-terminated polyisobutylenes, such as the products sold under the names Lubrizol 5603 and Lubrizol 2650 by the company Lubrizol. According to one preferred embodiment of the invention, the polymer sold under the name Lubrizol 5603 by the company Lubrizol, which is the diethylethanolamine salt of esterified succinic-terminated polyisobutylene (INCI name: Hydroxyethyldiethonium polyisobutenyl triethylaminosuccinate/diethylethanolamine), is used.
[0103]Another example of a polyisobutylene derivative that may be used in the invention is the product of reaction of maleic anhydride with polyisobutylene, such as the product sold under the name Glissopal SA by the company BASF.
[0104](5) Maleic anhydride copolymers comprising one or more maleic anhydride comonomers and one or more comonomers chosen from vinyl acetate, vinyl alcohol, vinylpyrrolidone, olefins containing from 2 to 20 carbon atoms and styrene, as described in patent EP 1 374 849, may also be mentioned as stabilizers.
[0105]According to the invention, the term “maleic anhydride copolymer” means any polymer obtained by copolymerization of one or more maleic anhydride comonomers and of one or more comonomers chosen from vinyl acetate, vinyl alcohol, vinylpyrrolidone, olefins containing from 2 to 20 carbon atoms, for instance octadecene, ethylene, isobutylene, diisobutylene, isooctylene, and styrene, the maleic anhydride comonomers being optionally partially or totally hydrolysed. Preferably, hydrophilic polymers will be used, i.e. polymers with a solubility in water of greater than or equal to 2 g/l.
[0106]Copolymers that are more particularly suitable for use in the invention are copolymers obtained by copolymerization of one or more maleic anhydride units, whose maleic anhydride units are in hydrolysed form, and preferentially in the form of alkaline salts, for example in the form of ammonium, sodium, potassium or lithium salts.
[0107]In one advantageous aspect of the invention, the copolymer has a mole fraction of maleic anhydride units of between 0.1 and 1 and more preferentially between 0.4 and 0.9.
[0108]According to one advantageous aspect of the invention, the mole ratio between the maleic anhydride unit equivalent and the oxidation-sensitive hydrophilic active agent ranges between 0.005 and 10 and preferentially between 0.01 and 1.
[0109]The weight-average molar mass of the maleic anhydride copolymers will advantageously be between 1000 and 500 000 and preferably between 1000 and 50 000.
[0110]Preferentially, a copolymer of styrene and of maleic anhydride in a 50/50 ratio will be used.
[0111]The styrene/maleic anhydride (50/50) copolymer, in the form of the ammonium salt at 30% in water, sold under the reference SMA1000HO by the company Atofina, or the styrene/maleic anhydride (50/50) copolymer, in the form of the sodium salt at 40% in water, sold under the reference SMA10000HNa® by the company Atofina, may be used, for example.
Photoprotective Agents
[0112]In order to improve the photostability of the compositions in accordance with the invention the said compositions may include one or more photoprotective agents.
[0113]The photoprotective agents in accordance with the invention are chosen from UVA-active and/or UVB-active organic and/or mineral UV-screening agents that are hydrophilic and/or lipophilic and/or insoluble in the commonly used cosmetic solvents.
[0114]The hydrophilic, lipophilic or insoluble organic UV-screening agents are chosen especially from anthranilates; dibenzoylmethane derivatives; cinnamic derivatives; salicylic derivatives; camphor derivatives; benzophenone derivatives; β,β-diphenyl-acrylate derivatives; triazine derivatives; benzo-triazole derivatives; benzalmalonate derivatives, especially those cited in U.S. Pat. No. 5,624,663; benzimidazole derivatives; imidazolines; bis-benzazolyl derivatives as described in patents EP 669 323 and U.S. Pat. No. 2,463,264; p-aminobenzoic acid (PABA) derivatives; methylenebis (hydroxyphenylbenzotriazole) derivatives as described in patent applications U.S. Pat. No. 5,237,071, U.S. Pat. No. 5,166,355, GB 2 303 549, DE 197 26 184 and EP 893 119; benzoxazole derivatives as described in patent applications EP 0 832 642, EP 1 027 883, EP 1 300 137 and DE 101 62 844; screening polymers and screening silicones such as those described especially in patent application WO 93/04665; α-alkylstyrene-based dimers, such as those described in patent application DE 198 55 649; 4,4-diarylbutadienes such as those described in patent applications EP 0 967 200, DE 197 46 654, DE 197 55 649, EP-A-1 008 586, EP 1 133 980 and EP 133 981, and mixtures thereof.
[0115]As examples of organic UV-screening agents, mention may be made of those denoted hereinbelow under their INCI name:
Para-Aminobenzoic Acid Derivatives:
PABA,
Ethyl PABA,
[0116]Ethyl dihydroxypropyl PABA,
Ethylhexyl dimethyl PABA sold in particular under the name “Escalol 507” by ISP,
Glyceryl PABA,
[0117]PEG-25 PABA sold under the name “Uvinul P25” by BASF.
Dibenzoylmethane Derivatives:
[0118]Butylmethoxydibenzoylmethane sold especially under the trade name “Parsol 1789” by Hoffmann LaRoche,
Isopropyldibenzoylmethane.
Salicylic Derivatives:
[0119]Homosalate sold under the name “Eusolex HMS” by Rona/EM Industries,
Ethylhexyl salicylate sold under the name “Neo Heliopan OS” by Haarmann and Reimer,
Dipropylene glycol salicylate sold under the name “Dipsal” by Scher,
TEA salicylate sold under the name “Neo Heliopan TS” by Haarmann and Reimer.
Cinnamic Derivatives:
[0120]Ethylhexyl methoxycinnamate sold in particular under the trade name “Parsol MCX” by Hoffmann LaRoche,
Isopropyl methoxycinnamate,
Isoamyl methoxycinnamate sold under the trade name “Neo Heliopan E 1000” by Haarmann and Reimer,
Cinoxate,
[0121]DEA methoxycinnamate,
Diisopropyl methylcinnamate,
Glyceryl ethylhexanoate dimethoxycinnamate.
β,β-Diphenylacrylate Derivatives:
[0122]Octocrylene sold in particular under the trade name “Uvinul N539” by BASF,
Etocrylene sold in particular under the trade name “Uvinul N35” by BASF.
Benzophenone Derivatives:
[0123]Benzophenone-1 sold under the trade name “Uvinul 400” by BASF,
Benzophenone-2 sold under the trade name “Uvinul D50” by BASF,
Benzophenone-3 or Oxybenzone sold under the trade name “Uvinul M40” by BASF,
Benzophenone-4 sold under the trade name “Uvinul MS40” by BASF,
Benzophenone-5,
[0124]Benzophenone-6 sold under the trade name “Helisorb 11” by Norquay,
Benzophenone-8 sold under the trade name “Spectra-Sorb UV-24” by American Cyanamid,
Benzophenone-9 sold under the trade name “Uvinul DS-49” by BASF,
Benzophenone-12
[0125]n-hexyl 2-(4-diethylamino-2-hydroxybenzoyl)benzoate.
Benzylidenecamphor Derivatives:
[0126]3-Benzylidenecamphor manufactured under the name “Mexoryl SD” by Chimex,
4-Methylbenzylidenecamphor sold under the name “Eusolex 6300” by Merck,
Benzylidenecamphorsulfonic acid manufactured under the name “Mexoryl SL” by Chimex,
Camphor benzalkonium methosulfate manufactured under the name “Mexoryl SO” by Chimex,
Terephthalylidenedicamphorsulfonic acid manufactured under the name “Mexoryl SX” by Chimex,
Polyacrylamidomethylbenzylidenecamphor manufactured under the name “Mexoryl SW” by Chimex.
Phenylbenzimidazole Derivatives:
[0127]Phenylbenzimidazolesulfonic acid sold in particular under the trade name “Eusolex 232” by Merck,
Disodium phenyl dibenzimidazole tetrasulfonate sold under the trade name “Neo Heliopan AP” by Haarmann and Reimer.
Phenylbenzotriazole Derivatives:
[0128]Drometrizole trisiloxane sold under the name “Silatrizole” by Rhodia Chimie,
Methylenebis(benzotriazolyl)tetramethylbutylphenol sold in solid form under the trade name “MIXXIM BE/100” by Fairmount Chemical, or in micronized form as an aqueous dispersion under the trade name “Tinosorb M” by Ciba Specialty Chemicals.
Triazine Derivatives:
[0129]bis-Ethylhexyloxyphenol methoxyphenyl triazine sold under the trade name Tinosorb S by Ciba Geigy, Ethylhexyl triazone sold in particular under the trade name Uvinul T150 by BASF,
Diethylhexyl butamido triazone sold under the trade name Uvasorb HEB by Sigma 3V,
- 2,4,6-tris(Dineopentyl 4′-aminobenzalmalonate)-s-triazine
the symmetrical triazine screening agents described in U.S. Pat. No. 6,225,467, patent application WO 2004/085 412 (see compounds 6 and 9) or the document “Symmetrical Triazine Derivatives” IP.COM Journal, IP.COM INC West Henrietta, N.Y., US (20 Sep. 2004), especially 2,4,6-tris(biphenyl)-1,3,5-triazines (in particular 2,4,6-tris(biphenyl-4-yl-1,3,5-triazine) and 2,4,6-tris(terphenyl)-1,3,5-triazine which is also mentioned in patent applications WO 06/035 000, WO 06/034 982, WO 06/034 991, WO 06/035 007, WO 2006/034 992 and WO 2006/034 985.
Anthranilic Derivatives:
[0131]Menthyl anthranilate sold under the trade name “Neo Heliopan MA” by Haarmann and Reimer.
Imidazoline Derivatives:
[0132]Ethylhexyldimethoxybenzylidenedioxoimidazoline propionate.
Benzalmalonate Derivatives:
[0133]Dineopentyl 4′-methoxybenzalmalonate
Polyorganosiloxane containing benzalmalonate functions, for instance Polysilicone-15, sold under the trade name “Parsol SLX” by Hoffmann LaRoche
4,4-Diarylbutadiene Derivatives:
[0000]- 1,1-Dicarboxy(2,2′-dimethylpropyl)-4,4-diphenyl-butadiene
Benzoxazole Derivatives:
[0135]2,4-bis[5-(1-dimethylpropyl)benzoxazol-2-yl(4-phenyl)imino]-6-(2-ethylhexyl)imino-1,3,5-triazine sold under the name Uvasorb K2A by Sigma 3V
and mixtures thereof.
[0136]The preferential organic UV-screening agents are chosen from:
- Ethylhexyl methoxycinnamate,
- Homosalate,
- Ethylhexyl salicylate,
- Octocrylene,
- Phenylbenzimidazolesulfonic acid,
- Benzophenone-3,
- Benzophenone-4,
- Benzophenone-5,
- n-Hexyl 2-(4-diethylamino-2-hydroxybenzoyl)benzoate,
- 4-Methylbenzylidenecamphor,
- Terephthalylidenedicamphorsulfonic acid,
- Disodium phenyldibenzimidazoletetrasulfonate,
- Ethylhexyl triazone,
- bis-Ethylhexyloxyphenol methoxyphenyl triazine,
- Diethylhexyl butamido triazone,
- 2,4,6-tris(Dineopentyl 4′-aminobenzalmalonate)-s-triazine,
- 2,4,6-tris(Diisobutyl 4′-aminobenzalmalonate)-s-triazine,
- 2,4,6-tris(Biphenyl-4-yl-1,3,5-triazine), 2,4,6-tris(Terphenyl)-1,3,5-triazine
[0155]Methylenebis(benzotriazolyl)tetramethylbutylphenol,
- Drometrizole trisiloxane,
- Polysilicone-15,
- Dineopentyl 4′-methoxybenzalmalonate,
- 1,1-Dicarboxy(2,2′-dimethylpropyl)-4,4-diphenyl-butadiene,
- 2,4-bis[5-1(Dimethylpropyl)benzoxazol-2-yl(4-phenyl)imino]-6-(2-ethylhexyl)imino-1,3,5-triazine, and mixtures thereof.
[0161]The inorganic screening agents are chosen from pigments (mean size of the primary particles: generally between 5 nm and 100 nm and preferably between 10 nm and 50 nm) of coated or uncoated metal oxides, for instance nanopigments of titanium oxide (amorphous or crystallized in rutile and/or anatase form), of iron oxide, of zinc oxide, of zirconium oxide or of cerium oxide, which are all UV photoprotective agents that are well known per se.
[0162]The pigments may be coated or uncoated.
[0163]The coated pigments are pigments that have undergone one or more surface treatments of chemical, electronic, mechanochemical and/or mechanical nature with compounds as described, for example, in Cosmetics & Toiletries, February 1990, Vol. 105, pp. 53-64, such as amino acids, beeswax, fatty acids, fatty alcohols, anionic surfactants, lecithins, sodium, potassium, zinc, iron or aluminium salts of fatty acids, metal alkoxides (of titanium or of aluminium), polyethylene, silicones, proteins (collagen, elastin), alkanolamines, silicon oxides, metal oxides or sodium hexametaphosphate.
[0164]As is known, silicones are organosilicon polymers or oligomers of linear or cyclic, branched or crosslinked structure, of variable molecular weight, obtained by polymerization and/or polycondensation of suitably functionalized silanes, and consist essentially of a repetition of main units in which the silicon atoms are linked together via oxygen atoms (siloxane bond), optionally substituted hydrocarbon-based radicals being directly attached via a carbon atom to the said silicon atoms.
[0165]The term “silicones” also includes the silanes required for their preparation, in particular alkyl silanes.
[0166]The silicones used for coating the pigments that are suitable for the present invention are preferably chosen from the group containing alkyl silanes, polydialkylsiloxanes and polyalkylhydrogenosiloxanes. Even more preferentially, the silicones are chosen from the group containing octyltrimethylsilane, polydimethylsiloxanes and polymethylhydrogenosiloxanes.
[0167]Needless to say, before being treated with silicones, the metal oxide pigments may have been treated with other surface agents, in particular with cerium oxide, alumina, silica, aluminium compounds or silicon compounds, or mixtures thereof.
[0168]The coated pigments are more particularly titanium oxides that have been coated:
[0169]with silica, such as the product Sunveil from the company Ikeda,
[0170]with silica and iron oxide, such as the product Sunveil F from the company Ikeda,
[0171]with silica and alumina, such as the products Microtitanium Dioxide MT 500 SA and Microtitanium Dioxide MT 100 SA from the company Tayca, Tioveil from the company Tioxide and Mirasun TiW 60 from the company Rhodia,
[0172]with alumina, such as the products Tipaque TTO-55 (B) and Tipaque TTO-55 (A) from the company Ishihara and UVT 14/4 from the company Kemira,
[0173]with alumina and aluminium stearate, such as the product Microtitanium Dioxide MT 100 T, MT 100 TX, MT 100 Z and MT-01 from the company Tayca, the products Solaveil CT-10 W and Solaveil CT 100 from the company Uniqema, and the product Eusolex T-AVO from the company Merck,
[0174]with silica, alumina and alginic acid, such as the product MT-100 AQ from the company Tayca,
[0175]with alumina and aluminium laurate, such as the product Microtitanium Dioxide MT 100 S from the company Tayca,
[0176]with iron oxide and iron stearate, such as the product Microtitanium Dioxide MT 100 F from the company Tayca,
[0177]with zinc oxide and zinc stearate, such as the product BR351 from the company Tayca,
[0178]with silica and alumina and treated with a silicone, such as the products Microtitanium Dioxide MT 600 SAS, Microtitanium Dioxide MT 500 SAS or Microtitanium Dioxide MT 100 SAS from the company Tayca,
[0179]with silica, alumina and aluminium stearate and treated with a silicone, such as the product STT-30-DS from the company Titan Kogyo,
[0180]with silica and treated with a silicone, such as the product UV-Titan X 195 from the company Kemira,
[0181]with alumina and treated with a silicone, such as the products Tipaque TTO-55 (S) from the company Ishihara or UV Titan M 262 from the company Kemira,
[0182]with triethanolamine, such as the product STT-65-S from the company Titan Kogyo,
[0183]with stearic acid, such as the product Tipaque TTO-55 (C) from the company Ishihara,
[0184]with sodium hexametaphosphate, such as the product Microtitanium Dioxide MT 150 W from the company Tayca.
[0185]Other titanium oxide pigments treated with a silicone are preferably TiO2 treated with octyltrimethylsilane and for which the mean size of the elementary particles is between 25 and 40 nm, such as the product sold under the trade name T 805 by the company Degussa Silices, TiO2 treated with a polydimethylsiloxane and for which the mean size of the elementary particles is 21 nm, such as the product sold under the trade name 70250 Cardre UF TiO2SI3 by the company Cardre, anatase/rutile TiO2 treated with a polydimethylhydrogenosiloxane and for which the mean size of the elementary particles is 25 nm, such as the product sold under the trade name Microtitanium Dioxide USP Grade Hydrophobic by the company Color Techniques.
[0186]The uncoated titanium oxide pigments are sold, for example, by the company Tayca under the trade names Microtitanium Dioxide MT 500 B or Microtitanium Dioxide MT 600 B, by the company Degussa under the name P 25, by the company Wackher under the name Transparent titanium oxide PW, by the company Myoshi Kasei under the name UFTR, by the company Tomen under the name ITS and by the company Tioxide under the name Tioveil AQ.
[0187]The uncoated zinc oxide pigments are, for example:
[0188]those sold under the name Z-Cote by the company Sunsmart;
[0189]those sold under the name Nanox by the company Elementis;
[0190]those sold under the name Nanogard WCD 2025 by the company Nanophase Technologies.
[0191]The coated zinc oxide pigments are, for example:
[0192]those sold under the name Zinc Oxide CS-5 by the company Toshibi (ZnO coated with polymethylhydrogenosiloxane);
[0193]those sold under the name Nanogard Zinc Oxide FN by the company Nanophase Technologies (as a 40% dispersion in Finsolv TN, C12-C15 alkyl benzoate);
[0194]those sold under the name Daitopersion ZN-30 and Daitopersion ZN-50 by the company Daito (dispersions in cyclopolymethylsiloxane/oxyethylenated polydimethylsiloxane, containing 30% or 50% of nanozinc oxides coated with silica and polymethylhydrogenosiloxane);
[0195]those sold under the name NFD Ultrafine ZNO by the company Daikin (ZnO coated with perfluoroalkyl phosphate and copolymer based on perfluoroalkylethyl as a dispersion in cyclopentasiloxane);
[0196]those sold under the name SPD-Zl by the company Shin-Etsu (ZnO coated with silicone-grafted acrylic polymer, dispersed in cyclodimethylsiloxane);
[0197]those sold under the name Escalol Z100 by the company ISP (alumina-treated ZnO dispersed in an ethylhexyl methoxycinnamate/PVP-hexadecene/methicone copolymer mixture);
[0198]those sold under the name Fuji ZNO-SMS-10 by the company Fuji Pigment (ZnO coated with silica and polymethylsilsesquioxane);
[0199]those sold under the name Nanox Gel TN by the company Elementis (ZnO dispersed at a concentration of 55% in C12-C15 alkyl benzoate with hydroxystearic acid polycondensate).
[0200]The uncoated cerium oxide pigments are sold under the name Colloidal Cerium Oxide by the company Rhone-Poulenc.
[0201]The uncoated iron oxide nanopigments are sold, for example, by the company Arnaud under the names Nanogard WCD 2002 (FE 45B), Nanogard Iron FE 45 EL AQ, Nanogard FE 4SR AQ and Nanogard WCD 2006 (FE 45R) or by the company Mitsubishi under the name TY-220.
[0202]The coated iron oxide pigments are sold, for example, by the company Arnaud under the names Nanogard WCD 2008 (FE 45B FN), Nanogard WCD 2009 (FE 45B 556), Nanogard FE 45 EL 345 and Nanogard FE 45 BL or by the company BASF under the name Transparent Iron Oxide.
[0203]Mention may also be made of mixtures of metal oxides, especially of titanium dioxide and of cerium dioxide, including the silica-coated equal-weight mixture of titanium dioxide and of cerium dioxide, sold by the company Ikeda under the name Sunveil A, and also the alumina, silica and silicone-coated mixture of titanium dioxide and of zinc dioxide, such as the product M 261 sold by the company Kemira, or the alumina, silica and glycerol-coated mixture of titanium dioxide and of zinc dioxide, such as the product M 211 sold by the company Kemira.
[0204]The photoprotective agents are generally present in the compositions containing dehydroascorbic acid or a polymer thereof and/or the compositions comprising ascorbic acid or a salt or derivative thereof in proportions ranging from 0.01% to 20% by weight relative to the total weight of the composition, and preferably ranging from 0.1% to 10% by weight relative to the total weight of the composition.
Additional Colouring Agents
[0205]In order to nuance the colour obtained and to better adapt it to the various types of skin tone, the compositions of the invention may also comprise one or more additional colouring agents.
[0206]The additional colouring agents may also be chosen especially from natural and synthetic direct dyes. They may be organic or mineral dyes.
[0207]The natural or synthetic liposoluble organic dyes are, for example, DC Red 17, DC Red 21, DC Red 27, DC Green 6, DC Yellow 11, DC Violet 2, DC Orange 5, Sudan red, carotenes (D-carotene or lycopene), xanthophylls (capsanthin, capsorubin or lutein), palm oil, Sudan brown, quinoline yellow, annatto and curcumin.
[0208]The natural or synthetic water-soluble dyes are, for example, FDC Red 4, DC Red 6, DC Red 22, DC Red 28, DC Red 30, DC Red 33, DC Orange 4, DC Yellow 5, DC Yellow 6, DC Yellow 8, FDC Green 3, DC Green 5, FDC Blue 1, betanin (beetroot), carmine, copper-containing chlorophylline, methylene blue, anthocyanins (enocyanin, black carrot, hibiscus or elder) and riboflavin.
[0209]The dyes may also be chosen from anthraquinones, caramel, carmine, carbon black, azulene blues, methoxalene, trioxalene, guajazulene, chamuzulene, rose Bengal, cosine 10B, cyanosin, daphinine, juglone, lawsone, extracts of fermented soya, of algae, of fungi or of microorganisms, flavylium salts not substituted in position 3, for instance those described in patent EP 1 172 091, extracts of Gesneria fulgens, Blechum procerum or Saxifraga and pigments that may be obtained by extraction with an organic or aqueous-organic solvent of a culture medium of micromycetes of the Monascus type.
[0210]These dyes may also be chosen from indole derivatives, for instance the monohydroxyindoles as described in patent FR 2 651 126 (i.e.: 4-, 5-, 6- or 7-hydroxyindole) or the dihydroxyindoles as described in patent EP-B-0 425 324 (i.e.: 5,6-dihydroxyindole, 2-methyl-5,6-dihydroxyindole, 3-methyl-5,6-dihydroxyindole or 2,3-dimethyl-5,6-dihydroxyindole).
[0211]These dyes may also be dyes obtained with compounds comprising at least one aromatic ring containing at least two hydroxyl groups (OH) borne by two consecutive carbon atoms of the aromatic ring and a catalytic system comprising a first constituent chosen from Mn(II) and/or Zn(II) salts and oxides, and mixtures thereof, and a second constituent chosen from alkali metal hydrogen carbonates, alkaline-earth metal hydrogen carbonates, and mixtures thereof, as described previously.
[0212]The additional colouring agents may also be chosen from particulate dyestuffs, which are preferably chosen from pigments, nacres or interference pigments, and glitter flakes.
[0213]The term “pigments” should be understood as meaning white or coloured, mineral or organic particles of any form, which are insoluble in the physiological medium and are intended to colour the composition.
[0214]The pigments may be white or coloured, and mineral and/or organic. Among the mineral pigments that may be mentioned are titanium dioxide, optionally surface-treated, zirconium oxide or cerium oxide, and also zinc oxide, iron (black, yellow or red) oxide or chromium oxide, manganese violet, ultramarine blue, chromium hydrate and ferric blue, and metal powders, for instance aluminium powder and copper powder.
[0215]Among the organic pigments that may be mentioned are carbon black, pigments of D&C type and lakes based on cochineal carmine or on barium, strontium, calcium or aluminium.
[0216]Mention may also be made of pigments with an effect, such as particles comprising a natural or synthetic, organic or mineral substrate, for example glass, acrylic resins, polyester, polyurethane, polyethylene terephthalate, ceramics or aluminas, the said substrate optionally being coated with metallic substances such as aluminium, gold, silver, platinum, copper or bronze, or metal oxides such as titanium dioxide, iron oxide or chromium oxide, and mixtures thereof.
[0217]For the purposes of the present invention, the term “interference particles or nacres” denotes any particle generally having a multilayer structure such that it allows the creation of a colour effect by interference of light rays, which diffract and scatter differently according to the nature of the layers. The colouring effects obtained are associated with the lamellar structure of these particles and are derived from the physical laws of thin film optics (see: Pearl lustre pigments—physical principles, properties, applications —R. Maisch, M. Weigand. Verlag Moderne Industrie). Thus, these particles may have colours that vary according to the angle of observation and the incidence of the light.
[0218]For the purposes of the present invention, a multilayer structure is intended to denote, without preference, a structure formed from a substrate coated with a single layer, or a structure formed from a substrate coated with at least two or even more consecutive layers.
[0219]The multilayer structure may thus comprise one or even at least two layers, each layer, independently or otherwise of the other layer(s), being made of at least one material chosen from the group consisting of the following materials: MgF2, CeF3, ZnS, ZnSe, Si, SiO2, Ge, Te, Fe2O3, Pt, Va, Al2O3, MgO, Y2O3, S2O3, SiO, HfO2, ZrO2, CeO2, Nb2O5, Ta2O5, TiO2, Ag, Al, Au, Cu, Rb, Ti, Ta, W, Zn, MOS2, cryolite, alloys and polymers, and combinations thereof.
[0220]Generally, the multilayer structure is of mineral nature.
[0221]More particularly, the interference particles under consideration according to the invention may be interference pigments, or alternatively natural or synthetic, monolayer or multilayer nacres, in particular formed from a natural substrate based, inter alia, on mica, which is covered with one or more layers of metal oxide.
[0222]The interference particles according to the invention are characterized in that 50% of the mass population has a diameter (d50) of less than 40 μm, more particularly less than 30 μm, especially less than 20 μm and in particular less than 15 μm, measured with a laser granulometer, for instance the Mastersizer 2000® machine from Malvern or the BI90+® machine from Brookhaven Instrument Corporation.
[0223]Nacres of mica/tin oxide/titanium oxide type, for instance those sold under the names Timiron Silk Blue®, Timiron Silk Red®, Timiron Silk Green®, Timiron Silk Gold® and Timiron Super Silk® sold by the company Merck, and mica/iron oxide/titanium oxide nacres, for instance Flamenco Satin Blue®, Flamenco Satin Red® and Flamenco Satin Violet® and Flamenco Orange 320C sold by the company Engelhard, and mixtures thereof, are most particularly suitable for the invention.
[0224]It is understood that the choice of these interference particles is made so as to be moreover compatible with the requirements in terms of lightness and saturation required for the compositions according to the invention. In general, these interference particles are present in an amount sufficient to obtain a homogeneous effect in terms of coloration while at the same time preserving the natural flesh tone of the skin.
[0225]More specifically, these pigments may be present in amounts ranging from 0.01% to 10% by weight and preferably ranging from 0.1% to 5% by weight relative to the total weight of the composition.
[0226]The additional colouring agents may also be chosen from fluorescers.
[0227]The term “fluorescer” means a substance which, under the effect of ultraviolet rays and/or visible light, re-emits in the visible region the portion of light that it has absorbed under the same colour as that which it naturally reflects. The naturally reflected colour is thus reinforced by the re-emitted colour and appears extremely bright.
[0228]Examples that may be mentioned include coloured polyamide and/or formaldehyde/benzoguanamine and/or melamine/formaldehyde/sulfonamide resins, from coloured aminotriazine/formaldehyde/sulfonamide co-condensates and/or from metallized polyester glitter flakes and/or mixtures thereof. These fluorescent pigments may also be present in the form of aqueous dispersions of fluorescent pigments.
[0229]Mention may also be made of the pink-coloured fluorescent aminotriazine/formaldehyde/sulfonamide co-condensate with a mean particle size of 3-4 microns sold under the trade name Fiesta Astral Pink FEX-1 and the blue-coloured fluorescent aminotriazine/formaldehyde/sulfonamide co-condensate with a mean particle size of 3-4.5 microns sold under the trade name Fiesta Comet Blue FTX-60 by the company Swada, or alternatively the yellow-coloured benzoguanamine/formaldehyde resin covered with formaldehyde/urea resin sold under the trade name FB-205 Yellow and the red-coloured benzoguanamine/formaldehyde resin covered with formaldehyde/urea resin sold under the trade name FB-400 Orange Red by the company UK Seung Chemical, and the orange-coloured polyamide resin sold under the trade name Flare 911 Orange 4 by the company Sterling Industrial Colors.
[0230]The fluorescent substances are preferably present in the composition in a content ranging from 0.1% to 20%, preferably from 0.1% to 15% and more preferably from 0.5% to 3% by weight relative to the total weight of the composition.
[0231]When the organic fluorescent substances are white, they are also known as optical brighteners.
[0232]The optical brightener has the effect of intensifying the radiance and reviving the shades of cosmetic compositions comprising them on application to the skin.
[0233]Among the optical brighteners that may be mentioned more particularly are stilbene derivatives, in particular polystyrylstilbenes and triazinestilbenes, coumarin derivatives, in particular hydroxycoumarins and aminocoumarins, oxazole, benzoxazole, imidazole, triazole and pyrazoline derivatives, pyrene derivatives and porphyrin derivatives, and/or mixtures thereof.
[0234]Such compounds are available, for example, under the trade names Tinopal SOP® and Uvitex OB® from the company Ciba Geigy.
[0235]The optical brighteners preferentially used are sodium 4,4′-bis[(4,6-dianilino-1,3,5-triazin-2-yl)amino]-stilbene-2,2′-disulfonate, 2,5-thiophenediylbis(5-tert-butyl-1,3-benzoxazole) and disodium 4,4′-distyrylbiphenylsulfonate, and/or mixtures thereof.
Fillers
[0236]The compositions of the invention may comprise at least one filler.
[0237]The term “fillers” should be understood as meaning colourless or white, mineral or synthetic particles of any form, which are insoluble in the medium of the composition irrespective of the temperature at which the composition is manufactured. These fillers serve especially to modify the rheology or the texture of the composition.
[0238]The fillers may be mineral or organic and of any form, platelet-shaped, spherical or oblong, irrespective of the crystallographic form (for example lamellar, cubic, hexagonal, orthorhombic, etc.). Mention may be made of talc, mica, silica, kaolin, polyamide (Nylon®) powder (Orgasol® from Atochem), poly-β-alanine powder and polyethylene powder, tetrafluoroethylene polymer (Teflon®) powder, lauroyllysine, starch, boron nitride, hollow polymer microspheres such as polyvinylidene chloride/acrylonitrile microspheres, for instance Expancel® (Nobel Industrie), acrylic acid copolymer microspheres (Polytrap® from the company Dow Corning) and silicone resin microbeads (for example Tospearls® from Toshiba), elastomeric polyorganosiloxane particles, precipitated calcium carbonate, magnesium carbonate, magnesium hydrogen carbonate, hydroxy-apatite, hollow silica microspheres (Silica Beads® from Maprecos), glass or ceramic microcapsules, and metal soaps derived from organic carboxylic acids containing from 8 to 22 carbon atoms and preferably from 12 to 18 carbon atoms, for example zinc stearate, magnesium stearate, lithium stearate, zinc laurate or magnesium myristate.
[0239]The compositions according to the invention may in particular comprise at least one matting filler, a soft-focus filler, a fluorescer, an abrasive or exfoliant filler, and mixtures thereof.
Matting Fillers
[0240]For the purposes of the invention, the term “matting filler” denotes a spherical or non-spherical, porous or non-porous particle with a refractive index of less than or equal to 2.2, especially less than or equal to 2 and in particular less than or equal to 1.8, preferably ranging from 1.3 to 1.6. The “matting fillers” according to the invention have a volume size comparable to that of the nacres used. The preferred size of the fillers is thus less than 15 μm measured with a laser granulometer, for instance the Mastersizer 2000 from Malvern or the BI90+ from Brookhaven Instrument Corporation.
[0241]In one preferential embodiment of the invention, the “matting fillers” are spherical.
[0242]In another preferential embodiment of the invention, the “matting fillers” are porous. In this case, the specific surface area of the particles, which may be related to the porosity, is greater than 10 m2/g and preferably greater than 50 m2/g.
[0243]The matting nature of the fillers according to the invention is defined by means of a gonioreflectometer measurement. To do this, the composition containing 5% fillers is spread onto a contrast card (Prufkarte type 24/5-250 cm2 sold by the company Erichsen) using a mechanical film spreader (wet thickness of 30 μm). The composition is then dried overnight at a temperature of 37° C., and the reflection is then measured using a gonioreflectometer. The result obtained is the ratio R between the specular reflection and the diffuse reflection. The value of R is proportionately smaller the greater the matting effect. The matting fillers according to the invention are those which, at a content of 5% in a cosmetic composition, give a value of R of less than 1 and preferably less than 0.75.
[0244]The matting effect of the agent and/or composition containing it may especially be evaluated using a gonioreflectometer, by measuring the ratio R between the specular reflection and the scattered reflection. A value of R of less than or equal to 2 generally reflects a matting effect.
[0245]The matting filler may especially be chosen from a rice starch or a corn starch, kaolinite, talc, a pumpkin seed extract, cellulose microbeads, plant fibres, synthetic fibres, in particular polyamide fibres, expanded acrylic copolymer microspheres, polyamide powders, silica powders, polytetrafluoroethylene powders, silicone resin powders, acrylic polymer powders, wax powders, polyethylene powders, powders of elastomeric crosslinked organopolysiloxane coated with silicone resin, talc/titanium dioxide/alumina/silica composite powders, amorphous mixed silicate powders, silicate particles and especially mixed silicate particles, and mixtures thereof.
[0246]The matting agent may especially be chosen from a rice starch or a corn starch, kaolinite, talc, a pumpkin seed extract, cellulose microbeads, plant fibres, synthetic fibres, in particular polyamide fibres, expanded acrylic copolymer microspheres, polyamide powders, silica powders, polytetrafluoroethylene powders, silicone resin powders, acrylic polymer powders, wax powders, polyethylene powders, powders of elastomeric crosslinked organopolysiloxane coated with silicone resin, talc/titanium dioxide/alumina/silica composite powders, amorphous mixed silicate powders, silicate particles and especially mixed silicate particles, and mixtures thereof.
[0247]Examples of matting agents that may especially be mentioned include:
[0248]rice or corn starch, in particular an aluminium starch octenyl succinate sold under the name Dry Flo® by the company National Starch;
[0252]a pumpkin seed extract as sold under the name Curbilene® by the company Indena;
[0253]cellulose microbeads as described in patent application EP 1 562 562;
[0254]fibres, such as silk fibre, cotton fibre, wool fibre, flax fibre, cellulose fibre extracted especially from wood, from vegetables or from algae, polyamide fibre (Nylon®), modified cellulose fibre, poly-p-phenyleneterephthamide fibre, acrylic fibre, polyolefin fibre, glass fibre, silica fibre, aramid fibre, carbon fibre, Teflon® fibre, insoluble collagen fibre, polyester fibre, polyvinyl chloride or polyvinylidene chloride fibre, polyvinyl alcohol fibre, polyacrylonitrile fibre, chitosan fibre, polyurethane fibre, polyethylene phthalate fibre, fibres formed from a mixture of polymers, resorbable synthetic fibres, and mixtures thereof described in patent application EP 1 151 742;
[0255]expanded acrylic copolymer microspheres such as those sold by the company EXPANCEL under the name Expancel 551®;
[0256]fillers with an optical effect as described in patent application FR 2 869 796, in particular:
- polyamide powders (Nylon®), for instance Nylon 12 particles of the Orgasol type from Arkema, with a mean size of 10 microns and a refractive index of 1.54,
- silica powders, for instance Silica beads SB150 from Miyoshi with a mean size of 5 microns and a refractive index of 1.45,
- polytetrafluoroethylene powders, for instance PTFE Ceridust 9205F from Clariant, with a mean size of 8 microns and a refractive index of 1.36,
- silicone resin powders, for instance the silicone resin Tospearl 145A from GE Silicone with a mean size of 4.5 microns and a refractive index of 1.41,
- acrylic copolymer powders, especially of polymethyl(meth)acrylate, for instance the PMMA particles Jurymer MBI from Nihon Junyoki, with a mean size of 8 microns and a refractive index of 1.49, or the Micropearl M100 and F 80 ED particles from the company Matsumoto Yushi-Seiyaku,
- wax powders, for instance the paraffin wax particles Microease 114S from Micropowders, with a mean size of 7 microns and a refractive index of 1.54,
- polyethylene powders, especially comprising at least one ethylene/acrylic acid copolymer, and in particular consisting of ethylene/acrylic acid copolymers, for instance the particles Flobeads EA 209 from Sumitomo (with a mean size of 10 microns and a refractive index of 1.48),
- elastomeric crosslinked organopolysiloxane powders coated with silicone resin, especially with silsesquioxane resin, as described, for example, in U.S. Pat. No. 5,538,793. Such elastomeric powders are sold under the names KSP-100, KSP-101, KSP-102, KSP-103, KSP-104 and KSP-105 by the company Shin-Etsu, and
- talc/titanium dioxide/alumina/silica composite powders such as those sold under the name Coverleaf® AR-80 by the company Catalyst & Chemicals,
- mixtures thereof,
- compounds that absorb and/or adsorb sebum as described in patent application FR 2 869 796. Mention may be made especially of:
- silica powders, for instance the porous silica microspheres sold under the name Silica Beads SB-700 sold by the company Myoshi, the products Sunsphere® H51, Sunsphere® H33 and Sunsphere® H53 sold by the company Asahi Glass; the polydimethylsiloxane-coated amorphous silica microspheres sold under the name SA Sunsphere® H-33 and SA Sunsphere® H-53 sold by the company Asahi Glass;
- amorphous mixed silicate powders, especially of aluminium and magnesium, for instance the product sold under the name Neusilin UFL2 by the company Sumitomo;
- polyamide (Nylon®) powders, for instance Orgasol® 4000 sold by the company Arkema, and
- acrylic polymer powders, especially of polymethyl methacrylate, for instance Covabead® LH85 sold by the company Wackherr; of polymethyl methacrylate/ethylene glycol dimethacrylate, for instance Dow Corning 5640 Microsponge® Skin Oil Adsorber sold by the company Dow Corning, or Ganzpearl® GMP-0820 sold by the company Ganz Chemical; of polyallyl methacrylate/ethylene glycol dimethacrylate, for instance Poly-Pore® L200 or Poly-Pore® E200 sold by the company Amcol; of ethylene glycol dimethacrylate/lauryl methacrylate copolymer, for instance Polytrap® 6603 sold by the company Dow Corning;
- silicate particles, such as alumina silicate;
- mixed silicate particles, such as:
- magnesium aluminium silicate particles, such as saponite or hydrated magnesium aluminium silicate with a sodium sulfate sold under the trade name Sumecton® by the company Kunimine;
- the magnesium silicate, hydroxyethylcellulose, black cumin oil, marrow oil and phospholipids complex or Matipure from Lucas Meyer, and
- mixtures thereof.
[0277]Preferred matting agents that may be used according to the invention include a pumpkin seed extract, a rice or corn starch, kaolinite, silicas, talc, polyamide powders, polyethylene powders, acrylic copolymer powders, expanded acrylic copolymer microspheres, silicone resin microbeads and mixed silicate particles, and mixtures thereof.
[0000]Fillers with a Soft-Focus Effect
[0278]These fillers may be any material capable of modifying and hiding wrinkles by virtue of their intrinsic physical properties. These fillers may especially modify wrinkles via a tensioning effect, a covering effect or a soft-focus effect.
[0279]Examples of fillers that may be given include the following compounds:
[0280]porous silica microparticles, for instance the Silica Beads® SB150 and SB700 from Miyoshi with a mean size of 5 μm; the series-H Sunspheres® from Asahi Glass, for instance Sunspheres H33, H51 with respective sizes of 3.5 and 5 μm;
[0281]hollow hemispherical silicone resin particles such as NLK 500®, NLK 506® and NLK 510® from Takemoto Oil and Fat, especially described in EP-A-1 579 849;
[0282]silicone resin powders, for instance the silicone resin Tospearl® 145A from GE Silicone, with a mean size of 4.5 μm;
[0283]acrylic copolymer powders, especially of polymethyl (meth)acrylate, for instance the PMMA particles Jurymer MEI® from Nihon Junyoki, with a mean size of 8 μm, the hollow PMMA spheres sold under the name Covabead® LH85 by the company Wackherr, and vinylidene/acrylonitrile/methylene methacrylate expanded microspheres sold under the name Expancel®;
[0284]wax powders, for instance the paraffin wax particles MicroEase® 114S from MicroPowders, with a mean size of 7 μm;
[0285]polyethylene powders, especially comprising at least one ethylene/acrylic acid copolymer for instance the Flobeads® EA 209 E from Sumitomo, with a mean size of 10 μm;
[0286]crosslinked elastomeric organopolysiloxane powders coated with silicone resin and especially with silsesquioxane resin, under the names KSP-100®, KSP-101, KSP-102, KSP-103®, KSP-104 and KSP-105 by the company Shin-Etsu;
[0287]talc/titanium dioxide/alumina/silica composite powders, for instance those sold under the name Coverleaf AR-80® by the company Catalyst & Chemicals;
[0288]talc, mica, kaolin, lauryl glycine, starch powders crosslinked with octenyl succinate anhydride, boron nitride, polytetrafluoroethylene powders, precipitated calcium carbonate, magnesium carbonate, magnesium hydrogen carbonate, barium sulfate, hydroxyapatite, calcium silicate, cerium dioxide and glass or ceramic microcapsules;
[0289]hydrophilic or hydrophobic, synthetic or natural, mineral or organic fibres such as silk fibres, cotton fibres, wool fibres, flax fibres, cellulose fibres extracted especially from wood, vegetables or algae, polyamide (Nylon®) fibres, modified cellulose fibres, poly-p-phenyleneterephthamide fibres, acrylic fibres, polyolefin fibres, glass fibres, silica fibres, aramid fibres, carbon fibres, polytetrafluoroethylene (Teflon®) fibres, insoluble collagen fibres, polyester fibres, polyvinyl chloride fibres, polyvinylidene chloride fibres, polyvinyl alcohol fibres, polyacrylonitrile fibres, chitosan fibres, polyurethane fibres, polyethylene phthalate fibres, fibres formed from a mixture of polymers, resorbable synthetic fibres, and mixtures thereof described in patent application EP 1 151 742;
[0290]spherical elastomeric crosslinked silicones, for instance Trefil E-505C® or E-506C® from Dow Corning;
[0291]abrasive fillers, which, via a mechanical effect, smooth out the skin microrelief, such as abrasive silica, for instance Abrasif SP from Semanez or nutshell powders (for example of apricot or walnut, from Cosmetochem).
[0292]The fillers with an effect on the signs of ageing are especially chosen from porous silica microparticles, hollow hemispherical silicone particles, silicone resin powders, acrylic copolymer powders, polyethylene powders, crosslinked elastomeric organopolysiloxane powders coated with silicone resin, talc/titanium dioxide/alumina/silica composite powders, precipitated calcium carbonate, magnesium carbonate, magnesium hydrogen carbonate, barium sulfate, hydroxyapatite, calcium silicate, cerium dioxide, glass or ceramic microcapsules, and silk fibres or cotton fibres, and mixtures thereof.
[0293]The filler may be a soft-focus filler.
[0294]The term “soft-focus” filler means a filler which in addition gives the complexion transparency and a hazy effect. Preferably, the soft-focus fillers have a mean particle size of less than or equal to 15 microns. These particles may be in any form and in particular may be spherical or non-spherical. These fillers are more preferably non-spherical.
[0295]The soft-focus fillers may be chosen from silica and silicate powders, especially alumina powder, powders of polymethyl methacrylate (PMMA) type, talc, silica/TiO2 or silica/zinc oxide composites, polyethylene powders, starch powders, polyamide powders, styrene/acrylic copolymer powders and silicone elastomers, and mixtures thereof.
[0296]Mention may be made in particular of talc with a number-average size of less than or equal to 3 microns, for example talc with a number-average size of 1.8 microns and especially the product sold under the trade name Talc P3® by the company Nippon Talc, Nylon® 12 powder, especially the product sold under the name Orgasol 2002 Extra D Nat Cos® by the company Atochem, silica particles 1% to 2% surface-treated with a mineral wax (INCI name: hydrated silica (and) paraffin) such as the products sold by the company Degussa, amorphous silica microspheres, such as the products sold under the name Sunsphere, for example of reference H-53® by the company Asahi Glass, and silica microbeads such as those sold under the name SB-700® or SB-150® by the company Miyoshi, this list not being limiting.
[0297]The concentration of these fillers with an effect on the signs of ageing in the compositions according to the invention may be between 0.1% and 40%, or even between 0.1% and 20% by weight, relative to the total weight of the composition.
Abrasive Fillers or Exfoliants
[0298]As exfoliants that may be used in rinse-out compositions according to the invention, examples that may be mentioned include exfoliants or scrubbing particles of mineral, plant or organic origin. Thus, polyethylene beads or powder, Nylon powder, polyvinyl chloride powder, pumice powder, ground apricot kernel or walnut husk, sawdust, glass beads and alumina, and mixtures thereof, may be used, for example.
[0299]Mention may also be made of Exfogreen from Solabia (bamboo extract), extracts of strawberry akenes (Strawberry Akenes from Greentech), peach kernel powder, apricot kernel powder, and finally, in the field of plant powders with an abrasive effect, mention may be made of cranberry kernel powder.
[0300]As abrasive fillers or exfoliants that are preferred according to the invention, mention will be made of peach kernel powder, apricot kernel powder, cranberry kernel powder, strawberry akene extracts and bamboo extracts.
[0301]The additional filler(s) used in the compositions according to the invention may represent preferably from 0.01% to 20% by weight and better still from 0.1% to 15%, and even better still, from 0.5 to 5% by weight relative to the total weight of the composition.
Cosmetic or Dermatological Active Agents
[0302]The compositions according to the invention may also comprise one or more additional cosmetic or dermatological active agents.
[0303]The additional active agents may be chosen especially from moisturizers, desquamating agents, agents for improving the barrier function, depigmenting agents, antioxidants, dermo-decontracting agents, anti-glycation agents, agents for stimulating the synthesis of dermal and/or epidermal macromolecules and/or for preventing their degradation, agents for stimulating fibroblast or keratinocyte proliferation and/or keratinocyte differentiation, agents for promoting the maturation of the horny envelope, NO-synthase inhibitors, peripheral benzodiazepine receptor (PBR) antagonists, agents for increasing the activity of the sebaceous glands, agents for stimulating the energy metabolism of cells, tensioning agents, liporestructuring agents, slimming agents, agents for promoting the cutaneous capillary circulation, calmatives and/or anti-irritants, sebo-regulators or anti-seborrhoeic agents, astringents, cicatrizing agents, anti-inflammatory agents and antiacne agents.
[0304]A person skilled in the art will select the said active agent(s) as a function of the effect desired on the skin, the lips, the nails, the eyelashes or the eyebrows.
[0305]Needless to say, a person skilled in the art will take care to select this or these optional additional compound(s), and/or the amount thereof, such that the advantageous properties of the corresponding composition according to the invention are not, or are not substantially, adversely affected by the envisaged addition.
[0306]For caring for and/or makingup aged skin, he will preferably choose at least one active agent chosen from moisturizers, desquamating agents, agents for improving the barrier function, depigmenting agents, antioxidants, dermo-decontracting agents, anti-glycation agents, agents for stimulating the synthesis of dermal and/or epidermal macromolecules and/or for preventing their degradation, agents for stimulating fibroblast or keratinocyte proliferation and/or keratinocyte differentiation, agents for promoting the maturation of the horny envelope, NO-synthase inhibitors, peripheral benzodiazepine receptor (PBR) antagonists, agents for increasing the activity of the sebaceous glands, agents for stimulating the energy metabolism of cells, lipo-restructuring agents, and agents for promoting the cutaneous capillary circulation for the area around the eyes.
[0307]For caring for and/or makingup greasy skin, a person skilled in the art will preferably choose at least one active agent chosen from desquamating agents, seboregulating agents or anti-seborrhoeic agents, and astringents.
[0308]At least one active agent chosen from anti-acne agents, cicatrizing agents and anti-inflammatory agents will preferably be chosen for caring for and/or makingup acne-prone skin.
[0309]For slimming care of the body, he will preferably choose an active agent chosen from slimming active agents and active agents for promoting the cutaneous capillary circulation.
[0310]Examples of such compounds are described below.
1. Moisturizers or Humectants
[0311]Moisturizers or humectants that may especially be mentioned include glycerol and derivatives thereof, urea and derivatives thereof, especially Hydrovance® sold by National Starch, lactic acid, hyaluronic acid, AHAs, EHAs, sodium pidolate, xylitol, serine, sodium lactate, ectoin and derivatives thereof, chitosan and derivatives thereof, collagen, plankton, an extract of Imperata cylindra sold under the name Moist 24® by the company Sederma, acrylic acid homopolymers, for instance Lipidure-HM® from NOF Corporation, beta-glucan and in particular sodium carboxymethyl beta-glucan from Mibelle-AG-Biochemistry; a mixture of passionflower oil, apricot oil, corn oil and rice bran oil sold by Nestle under the name NutraLipids®; a C-glycoside derivative such as those described in patent application WO 02/051 828 and in particular C-β-D-xylopyranoside-2-hydroxypropane in the form of a solution containing 30% by weight of active material in a water/propylene glycol mixture (60/40% by weight) such as the product sold by Chimex under the trade name Mexoryl SBB®; an oil of musk rose sold by Nestle; an extract of the microalga Prophyridium cruentum enriched with zinc, sold by Vincience under the name Algualane Zinc®; spheres of collagen and of chondroitin sulfate of marine origin (Atelocollagen) sold by the company Engelhard Lyon under the name Marine Filling Spheres; hyaluronic acid spheres such as those sold by the company Engelhard Lyon.
[0312]The moisturizer that will preferably be used is chosen from urea and derivatives thereof, especially Hydrovance® sold by National Starch, hyaluronic acid, AHAs, BHAs, acrylic acid homopolymers, for instance Lipidure-HM® from NOF Corporation, beta-glucan and in particular sodium carboxymethyl beta-glucan from Mibelle-AG-Biochemistry; a mixture of passionflower oil, apricot oil, corn oil and rice bran oil sold by Nestlé under the name NutraLipids®; a C-glycoside derivative such as those described in patent application WO 02/051 828 and in particular C-β-D-xylopyranoside-2-hydroxypropane in the form of a solution containing 30% by weight of active material in a water/propylene glycol mixture (60/40% by weight) such as the product sold by Chimex under the trade name Mexoryl SBB®; an oil of musk rose sold by Nestlé; an extract of the microalga Prophyridium cruentum enriched with zinc, sold by Vincience under the name Algualane Zinc®; spheres of collagen and of chondroitin sulfate of marine origin (Atelocollagen) sold by the company Engelhard Lyon under the name Marine Filling Spheres; hyaluronic acid spheres such as those sold by the company Engelhard Lyon.
2. Desquamating Agents
[0313]The term “desquamating agent” means any compound capable of acting:
[0314]either directly on desquamation by promoting exfoliation, such as β-hydroxy acids (BHAs), in particular salicylic acid and derivatives thereof (including 5-n-octanoylsalicylic acid, also known as capryloyl salicylic acid as the INCI name); α-hydroxy acids (AHAs), such as glycolic acid, citric acid, lactic acid, tartaric acid, malic acid or mandelic acid; 8-hexadecene-1,16-dicarboxylic acid or 9-octadecenedioic acid; urea and derivatives thereof; gentisic acid and derivatives thereof; oligofucoses; cinnamic acid; Saphora japonica extract; resveratrol, and certain jasmonic acid derivatives;
[0315]or on the enzymes involved in the desquamation or degradation of corneodesmosomes, glycosidases, stratum corneum chymotryptic enzyme (SCCE) or other proteases (trypsin, chymotrypsin-like) Mention may be made of aminosulfonic compounds and in particular 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (HEPES); 2-oxothiazolidine-4-carboxylic acid (procysteine) and derivatives thereof; derivatives of α-amino acids of glycine type (as described in EP-0 852 949, and also sodium methyl glycine diacetate sold by BASF under the trade name Trilon M); honey; sugar derivatives such as O-octanoyl-6-D-maltose and N-acetylglucosamine.
[0316]As other desquamating agents that may be used in the composition according to the invention, mention may be made of:
[0317]oligofructoses, EDTA and derivatives thereof, laminaria extracts, O-linoleyl-6D-glucose, (3-hydroxy-2-pentylcyclopentyl)acetic acid, glycerol trilactate, O-octanyl-6′-D-maltose, S-carboxymethylcysteine, siliceous derivatives of salicylate such as those described in patent EP 0 796 861, oligofucases such as those described in patent EP 0 218 200, 5-acyl salicylic acid salts, active agents with effects on transglutaminase, as in patent EP 0 899 330,
[0318]extract of the flowers of ficus Opuntia indica (Exfolactive® from Silab),
[0319]8-hexadecene-1,16-dicarboxylic acid,
[0320]esters of glucose and of vitamin F, and
[0322]Preferred desquamating agents that may be mentioned include β-hydroxy acids such as 5-n-octanoyl salicylic acid; urea; glycolic acid, citric acid, lactic acid, tartaric acid, malic acid or mandelic acid; 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (HEPES); extract of Saphora japonica; honey; N-acetyl glucosamine; sodium methyl glycine diacetate, and mixtures thereof.
[0323]Even more preferentially, a desquamating agent chosen from 5-n-octanoyl salicylic acid; urea; 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (HEPES); extract of Saphora japonica; honey; N-acetyl glucosamine; sodium methyl glycine diacetate, and mixtures thereof, will be used in the compositions of the invention.
3. Agents for Improving the Barrier Function
[0324]As agents for improving the barrier function, mention may be made especially of arginine, serine, an extract of Thermus thermophilus such as Venuceane® from Sederma, an extract of the rhizome of wild yam (Dioscorea villosa) such as Actigen Y® from Active Organics, plankton extracts, for instance Omega Plankton® from Secma, yeast extracts, for instance Relipidium® from Coletica, a chestnut extract such as Recoverine® from Silab, a cedar bud extract such as Gatuline Zen® from Gattefosse, sphingosines, for instance salicyloyl sphingosine sold under the name Phytosphingosine® SLC by the company Degussa, a mixture of xylitol, polyxylityl glycoside and xylitan, for instance Aquaxyl® from SEPPIC, extracts of Solanacea plants, for instance Lipidessence® from Coletica, omega-3 unsaturated oils such as musk rose oils, and mixtures thereof.
[0325]Mention may also be made especially of ceramides or derivatives thereof, in particular ceramides of type 2 (for instance N-oleoyldihydrosphingosine), of type 3 (for instance stearoyl-4-hydroxysphinganine, as the INCI name) and of type 5 (for instance N-2-hydroxypalmitoyldihydrosphingosine, having the INCI name: hydroxypalmitoyl sphinganine), sphingoid-based compounds, glycosphingolipids, phospholipids, cholesterol and derivatives thereof, phytosterols, essential fatty acids, diacylglycerol, 4-chromanone and chromone derivatives, petroleum jelly, lanolin, shea butter, cocoa butter and PCA salts.
[0326]As preferred agents having a restructuring effect on the barrier function, mention will be made of an extract of Thermus thermophilus, an extract of wild yam rhizome (Dioscorea villosa), a yeast extract, a chestnut extract, a cedar bud extract, arginine, serine, ceramides especially of type 3 and 5; and mixtures thereof.
[0327]Serine, arginine or a mixture thereof will preferably be used.
4. Depigmenting Agents
[0328]Depigmenting agents that may especially be mentioned include alpha and beta arbutin, ferulic acid, lucinol and derivatives thereof, kojic acid, resorcinol and derivatives thereof, tranexamic acid and derivatives thereof, gentisic acid, homogentisate, methyl gentisate or homogentisate, dioic acid, calcium D-pantheteine sulfonate, lipoic acid, ellagic acid, vitamin B3, linoleic acid and derivatives thereof, ceramides and homologues thereof, plant derivatives, for instance camomile, bearberry, the aloe family (vera, ferox, bardensis), mulberry or skullcap; a kiwi fruit (Actinidia chinensis) juice sold by Gattefosse, an extract of Paeonia suffruticosa root, such as the product sold by the company Ichimaru Pharcos under the name Botanpi Liquid B®, an extract of brown sugar (Saccharum officinarum), such as the extract of molasses sold by the company Taiyo Kagaku under the name Molasses Liquid, without this list being exhaustive.
[0329]Preferred depigmenting agents that will be used include alpha and beta arbutin, ferulic acid, kojic acid, resorcinol and derivatives thereof, calcium D-pantheteine sulfonate, lipoic acid, ellagic acid, vitamin B3, a kiwi fruit (Actinidia chinensis) juice sold by Gattefosse, and an extract of Paeonia suffruticosa root, such as the product sold by the company Ichimaru Pharcos under the name Botanpi Liquid B®.
5. Antioxidants
[0330]Mention may be made especially of tocopherol and esters thereof, in particular tocopheryl acetate; ferulic acid; serine; ellagic acid, phloretin, polyphenols, tannins, tannic acid, epigallocatechins and natural extracts containing them, anthocyans, rosemary extracts, olive leaf extracts, for instance those from the company Silab, green tea extracts, resveratrol and derivatives thereof, ergothioneine, N-acetylcysteine, an extract of the brown alga Pelvetia caniculata, for instance Pelvetiane® from Secma, chlorogenic acid, biotin, chelating agents, such as BHT and BHA, N,N′-bis(3,4,5-trimethoxybenzyl)ethylenediamine and salts thereof; idebenone, plant extracts, for instance Pronalen Bioprotect™ from the company Provital; coenzyme Q10, bioflavonoids, SODs, phytanetriol, lignans, melatonin, pidolates, glutathione, caprylyl glycol, Totarol™ or extract of Podocarpus totara containing Totarol (totara-8,11,13-trienol or 2-phenanthrenol, 4b,5,6,7,8,8a,9,10-octahydro-4-b,8,8-trimethyl-1-(1-methylethyl)-; a jasmine extract such as the product sold by Silab under the name Helisun®; hesperitin laurate such as Flavagrum PEG® from the company Engelhard Lyon; an extract of Paeonia suffruticosa root, such as the product sold by the company Ichimaru Pharcos under the name Botanpi Liquid B®; an extract of lychee such as the extract of lychee pericarp sold by the company Cognis under the name Litchiderm LS 9704®, an extract of pomegranate fruit (Punica granatum), such as the product sold by the company Draco Natural Products.
[0331]Other anti-ageing agents that may be mentioned include DHEA and derivatives thereof, boswellic acid, rosemary extracts, carotenoids (β-carotene, zeaxanthin and lutein), cysteic acid, copper derivatives and jasmonic acid.
[0332]Preferred antioxidants that will especially be used include ferulic acid; serine; phloretin, an extract of pomegranate, biotin, chelating agents such as BHT, BHA, N,N′-bis(3,4,5-trimethoxybenzyl)ethylenediamine and salts thereof, caprylyl glycol, Totarol™, a jasmine extract such as the product sold by Silab under the name Helisun®; hesperitin laurate such as Flavagrum PEG® from the company Engelhard Lyon; an extract of Paeonia suffruticosa root, such as the product sold by the company Ichimaru Pharcos under the name Botanpi Liquid B®.
6. Dermo-Relaxing or Dermo-Decontracting Agents
[0333]Examples that may be mentioned include manganese gluconate and other salts, adenosine, alverine citrate and salts thereof, glycine, an extract of Iris pallida, a hexapeptide (Argeriline R from Lipotec) or sapogenins, for instance wild yam and the carbonyl amines described in patent application EP 1 484 052. Examples of sapogenins that may be mentioned include those described in patent application WO 02/47650, in particular wild yam, the diosgenin extracted especially from Dioscorea opposita or any extract naturally containing or containing after treatment one or more sapogenins (wild yam rhizome, agave leaf, which contains hecogenin and tigogenin, extracts of Liliacea plants and more particularly yucca or smilax containing smilagenin and sarsapogenin, or sarsaparilla) or Actigen Y from the company Actives Organics, or ginger.
[0334]Mention may also be made of DMAE (dimethyl MEA), extracts of sea fennel, of rockrose, of helichrysum, of aniseed, of paracress, and an extract of Acmella oleracea, for instance Gatuline® from Gattefossé.
[0335]Preferred dermo-relaxing agents that will be mentioned include adenosine, manganese gluconate, wild yam, sea fennel, glycine and alverine.
7. Anti-Glycation Agents
[0336]The term “anti-glycation agent” means a compound that prevents and/or reduces the glycation of skin proteins, in particular dermal proteins such as collagen.
[0337]Anti-glycation agents that may especially be mentioned include extracts of plants of the Ericacea family, such as an extract of blueberry (Vaccinium angustifolium or Vaccinium myrtillus), for example the product sold under the name Blueberry Herbasol Extract PG by the company Cosmetochem, ergothioneine and derivatives thereof, hydroxystilbenes and derivatives thereof, such as resveratrol and 3,3′,5,5′-tetrahydroxystilbene (these anti-glycation agents are described in patent applications FR 2 802 425, FR 2 810 548, FR 2 796 278 and FR 2 802 420, respectively), dihydroxystilbenes and derivatives thereof, polypeptides of arginine and of lysine such as the product sold under the name Amadorine® by the company Solabia, carsinine hydrochloride (sold by Exsymol under the name Alistin®), an extract of Helianthus annuus, for instance Antiglyskin® from Silab, wine extracts such as the extract of powdered white wine on a maltodextrin support sold under the name Vin blanc deshydraté 2F by the company Givaudan, thioctic acid (or alpha-lipoic acid), a mixture of extract of bearberry and of marine glycogen, for instance Aglycal LS 8777® from Laboratoires Sérobiologiques, and an extract of black tea, for instance Kombuchka® from Sederma, and mixtures thereof.
[0338]Preferred anti-glycation agents that will be mentioned include extracts of blueberry (Vaccinium myrtillus) and extracts of black tea.
[0000]8. Agents for Stimulating the Synthesis of Dermal and/or Epidermal Macromolecules and/or for Preventing their Degradation
[0339]Among the active agents for stimulating the dermal macromolecules or for preventing their degradation, mention may be made of those acting:
[0340]either on collagen synthesis, such as extracts of Centella asiatica, asiaticosides and derivatives thereof; ascorbic acid or vitamin C and derivatives thereof; synthetic peptides such as iamin, biopeptide CL or palmitoyl oligopeptide sold by the company Sederma; peptides extracted from plants, such as the soybean hydrolysate sold by the company Coletica under the trade name Phytokine®; rice peptides such as Nutripeptide® from Silab, methylsilanol mannuronate such as Algisium C® sold by Exsymol; plant hormones such as auxins and lignans; folic acid; and an extract of Medicago sativa (alfalfa) such as the product sold by Silab under the name Vitanol®; a peptide extract of hazelnut such as the product sold by the company Solabia under the name Nuteline C®; and arginine;
[0341]or on the inhibition of collagen degradation, in particular agents acting on the inhibition of metalloproteases (MMP) more particularly such as MMP 1, 2, 3 and 9. Mention may be made of: retinoids and derivatives, extracts of Medicago sativa such as Vitanol® from Silab, an extract of Aphanizomenon flosaquae (Cyanophyceae) sold under the name Lanablue® by Atrium Biotechnologies, oligopeptides and lipopeptides, lipoamino acids, the malt extract sold by the company Coletica under the trade name Collalift®; blueberry or rosemary extracts; lycopene; isoflavones, derivatives thereof or plant extracts containing them, in particular extracts of soybean (sold, for example, by the company Ichimaru Pharcos under the trade name Flavosterone SB®), of red clover, of flax or of kakkon; an extract of lychee such as the extract of lychee pericarp sold by the company Cognis under the name Litchiderm LS 9704®; Dipalmitoyl Hydroxyproline sold by SEPPIC under the name Sepilift DPHP®: Baccharis genistelloides or Baccharine sold by Silab, an extract of moring a such as Arganyl LS 9781® from Cognis; the sage extract described in patent application FR-A-2 812 544 from the Labiatae family (Salvia officinalis from the company Flacksmann), an extract of rhododendron, a blueberry extract, and an extract of Vaccinium myrtillus such as those described in patent application FR-A-2 814 950;
[0342]or on the synthesis of molecules belonging to the elastin family (elastin and fibrillin), such as: retinol and derivatives, in particular retinyl palmitate; the extract of Saccharomyces cerevisiae sold by the company LSN under the trade name Cytovitin®; and the extract of the alga Macrocystis pyrifera sold by the company Secma under the trade name Kelpadelie®; a peptide extract of hazelnut such as the product sold by the company Solabia under the trade name Nuteline C®;
[0343]or on inhibition of elastin degradation, such as the peptide extract of seeds of Pisum sativum sold by the company LSN under the trade name Parelastyl®; heparinoids; and the N-acylamino acid compounds described in patent application WO 01/94381, such as {2-[acetyl(3-trifluoromethylphenyl)amino]-3-methylbutyrylamino}acetic acid, also known as N-[N-acetyl, N′-(3-trifluoromethyl)phenylvalyl]glycine, or N-acetyl-N-[3-(trifluoromethyl)phenyl]valylglycine or acetyl trifluoromethylphenylvalylglycine, or an ester thereof with a C1-C6 alcohol; an extract of rice peptides such as Colhibin® from Pentapharm, or an extract of Phyllanthus emblica such as Emblica® from Rona;
[0344]or on the synthesis of glycosaminoglycans, such as the product of fermentation of milk with Lactobacillus vulgaris, sold by the company Brooks under the trade name Biomin Yoghurt®; the extract of the brown alga Padina pavonica sold by the company Alban Müller under the trade name HSP3®; the Saccharomyces cerevisiae extract available especially from the company Silab under the trade name Firmalift® or from the company LSN under the trade name Cytovitin®; an extract of Laminaria ochroleuca such as Laminaine® from Secma; essence of Mamaku from Lucas Meyer, and an extract of Cress (Odraline® from Silab);
[0345]or on the synthesis of fibronectin, such as the extract of the zooplankton Salina sold by the company Seporga under the trade name GP4G®; the yeast extract available especially from the company Alban Müller under the trade name Drieline®; and the palmitoyl pentapeptide sold by the company Sederma under the trade name Matrixyl®.
[0346]Among the active agents for stimulating epidermal macromolecules, such as fillagrin and keratins, mention may be made especially of the extract of lupin sold by the company Silab under the trade name Structurine®; the extract of Fagus sylvatica beech buds sold by the company Gattefosse under the trade name Gatuline® RC; and the extract of the zooplankton Salina sold by the company Seporga under the trade name GP4G®; the copper tripeptide from Procyte; a peptide extract of Voandzeia substerranea such as the product sold by the company Laboratoires Sérobiologiques under the trade name Filladyn LS 9397®.
[0347]Preferably, an active agent that stimulates the synthesis of dermal and/or epidermal macromolecules and/or that prevents their degradation, chosen from agents for stimulating the synthesis of glycosaminoglycans, agents for inhibiting elastin degradation, agents for stimulating fibronectin synthesis, agents for stimulating the synthesis of epidermal macromolecules, and mixtures thereof, will be used.
[0348]Even more preferentially, an active agent that stimulates the synthesis of the glycosaminoglycans, chosen from an extract of the brown alga Padina pavonica, an extract of Saccharomyces cerevisiae, an extract of Laminaria ochroleuca, essence of Mamaku, and an extract of cress, and mixtures thereof, will be used.
[0349]As preferred active agents for stimulating the synthesis of dermal and/or epidermal macromolecules and/or for preventing their degradation, mention may be made of:
[0000]synthetic peptides such as iamin, the biopeptide CL or palmitoyloligopeptide sold by the company Sederma; peptides extracted from plants, such as the soybean hydrolysate sold by the company Coletica under the trade name Phytokine®; rice peptides such as Nutripeptide® from Silab, methylsilanol mannuronate such as Algisium C® sold by Exsymol; folic acid; an extract of Medicago sativa (alfalfa), such as the product sold by Silab under the name Vitanol®; a peptide extract of hazelnut, such as the product sold by the company Solabia under the name Nuteline C®; arginine; an extract of Aphanizomenon flos-aquae (Cyanophyceae) sold under the name Lanablue® by Atrium Biotechnologies, the malt extract sold by the company Coletica under the trade name Collalift®, lycopene; an extract of lychee; an extract of moring a such as Arganyl LS 9781® from Cognis; an extract of Vaccinium myrtillus such as those described in patent application FR-A-2 814 950; retinol and derivatives thereof, in particular retinyl palmitate; the extract of Saccharomyces cerevisiae sold by the company LSN under the trade name Cytovitin®; a peptide extract of hazelnut such as the product sold by the company Solabia under the name Nuteline C®; {2-[acetyl(3-trifluoromethylphenyl)amino]-3-methylbutyrylamino}-acetic acid, also known as N-[N-acetyl, N′-(3-trifluoromethyl)phenylvalyl]glycine, or N-acetyl-N-[3-(trifluoromethyl)phenyl]valylglycine or acetyl trifluoromethylphenylvalylglycine, or an ester thereof with a C1-C6 alcohol; an extract of rice peptides such as Colhibin® from Pentapharm, or an extract of Phyllanthus emblica such as Emblica® from Rona; the extract of the brown alga Padina pavonica sold by the company Alban Müller under the trade name HSP3®; the extract of Saccharomyces cerevisiae available especially from the company Silab under the trade name Firmalift® or from the company LSN under the trade name Cytovitin®; an extract of Laminaria ochroleuca such as Laminaine® from Secma; the essence of Mamaku from Lucas Meyer, the extract of lupin sold by the company Silab under the trade name Structurine®; the extract of Fagus sylvatica beech buds sold by the company Gattefosse under the trade name Gatuline® RC.
9. Agents for Stimulating Fibroblast or Keratinocyte Proliferation and/or Keratinocyte Differentiation
[0350]The agents for stimulating fibroblast proliferation that may be used in the composition according to the invention may be chosen, for example, from plant proteins or polypeptides, extracted especially from soybean (for example a soybean extract sold by the company LSN under the name Eleseryl SH-VEG 8® or sold by the company Silab under the trade name Raffermine®); an extract of hydrolysed soybean proteins such as Ridulisse® from Silab; and plant hormones such as gibberellins and cytokinins; a peptide extract of hazelnut such as the product sold by the company Solabia under the name Nuteline C®.
[0351]Preferably, an agent that promotes keratinocyte proliferation and/or differentiation will be used.
[0352]The agents for stimulating keratinocyte proliferation that may be used in the composition according to the invention especially comprise adenosine; phloroglucinol, the extract of Hydrangea macrophylla leaves, for instance Amacha Liquid E® from Ichimaru Pharcos, a yeast extract such as Stimoderm® from CLR; the extract of Larrea divaricata such as Capislow® from Sederma, mixtures of extract of papaya, of olive leaves and of lemon, such as Xyleine® from Vincience, retinol and esters thereof, including retinyl palmitate, phloroglucinol, the nut cake extracts sold by the Gattefosse and the extracts of Solanum tuberosum such as Dermolectine® sold by Sederma.
[0353]Among the agents for stimulating keratinocyte differentiation are, for example, minerals such as calcium; sea fennel, a peptide extract of lupin, such as the product sold by the company Silab under the trade name Structurine®; sodium beta-sitosteryl sulfate, such as the product sold by the company Seporga under the trade name Phytocohesine®; and a water-soluble extract of corn, such as the product sold by the company Solabia under the trade name Phytovityl®; a peptide extract of Voandzeia substerranea such as the product sold by the company Laboratoires Sérobiologiques under the trade name Filladyn LS 9397®; and lignans such as secoisolariciresinol, and retinol and esters thereof, including retinyl palmitate.
[0354]As agents for stimulating keratinocyte proliferation and/or differentiation, mention may also be made of oestrogens such as oestradiol and homologues; cytokines.
[0355]As preferred active agents for stimulating fibroblast or keratinocyte proliferation and/or keratinocyte differentiation, mention will be made of plant proteins or polypeptides, extracted especially from soybean (for example a soybean extract sold by the company LSN under the name Eleseryl SH-VEG 8® or sold by the company Silab under the trade name Raffermine®); an extract of hydrolysed soybean proteins such as Ridulisse® from Silab; a peptide extract of hazelnut such as the product sold by the company Solabia under the name Nuteline C®; adenosine; phloroglucinol, a yeast extract such as Stimoderm® from CLR; a peptide extract of lupin such as the product sold by the company Silab under the trade name Structurine®; a water-soluble corn extract, such as the product sold by the company Solabia under the trade name Phytovityl®; a peptide extract of Voandzeia substerranea, such as the product sold by the company Laboratoires Sérobiologiques under the trade name Filladyn LS 9397®; retinol and esters thereof, including retinyl palmitate.
[0000]10. Agents for Promoting the Maturation of the Horny Envelope Agents that participate in the maturation of the horny envelope, which becomes impaired with age and induces a decrease in transglutaminase activity, may be used in the compositions of the invention. Examples that may be mentioned include urea and derivatives thereof and in particular Hydrovance® from National Starch and the other active agents mentioned in L'Oreal patent application FR 2 877 220 (unpublished).
11. NO-Synthase Inhibitors
[0356]The agent with an inhibitory action on NO synthase may be chosen from OPCs (procyannidol oligomers); plant extracts of the species Vitis vinifera sold especially by the company Euromed under the name “Leucocyanidines de raisins extra”, or by the company Indena under the name Leucoselect®, or finally by the company Hansen under the name “Extrait de marc de raisin”; plant extracts of the species Olea europaea preferably obtained from olive tree leaves and sold especially by the company Vinyals in the form of a dry extract, or by the company Biologia & Technologia under the trade name Eurol® DT; and plant extracts of the species Gingko biloba, preferably a dry aqueous extract of this plant sold by the company Beaufour under the trade name “Ginkgo biloba extrait standard”, and mixtures thereof.
12. Peripheral Benzodiazepine Receptor (PBR) Antagonists
[0357]Mention may be made, for example, of 1-(2-chlorophenyl)-N-(1-methylpropyl)-3-isoquinoline carboxamide; the compounds described in patent applications WO 03/030 937 and WO 03/068 753, pyridazino[4,5-b]indole-1-acetamide derivatives of general formula (VII) as described in document WO 00/44384.
13. Agents for Increasing the Activity of the Sebaceous Glands
[0358]Mention may be made, for example, of methyl dehydrojasmonate, hecogenin, hedione and O-linoleyl-6D-glucose, and mixtures thereof.
14. Agents for Stimulating the Energy Metabolism of Cells
[0359]The active agent for stimulating the energy metabolism of cells may be chosen, for example, from biotin, an extract of Saccharomyces cerevisiae such as Phosphovital® from Sederma, the mixture of sodium, manganese, zinc and magnesium salts of pyrrolidonecarboxylic acid, for instance Physiogenyl® from Solabia, a mixture of zinc, copper and magnesium gluconate, such as Sepitonic M3® from SEPPIC, and mixtures thereof; a beta-glucan derived from Saccharomyces cerevisiae, such as the product sold by the company Mibelle AG Biochemistry.
15. Tensioning Agents
[0360]The term “tensioning agent” that may be used according to the invention means compounds liable to have a tensioning effect, i.e. being able to make the skin taut.
[0361]According to the invention, the term “tensioning agent” generally means any polymer that is soluble or dispersible in water at a temperature ranging from 25° C. to 50° C. at a concentration of 7% by weight in water or at the maximum concentration at which a medium of uniform appearance is formed and producing at this concentration of 7% or at this maximum concentration in water a shrinkage of more than 15% in the test described below.
[0362]The maximum concentration at which a medium of uniform appearance forms is determined to within ±10% and preferably to within ±5%.
[0363]The expression “medium of uniform appearance” means a medium that does not contain any aggregates that are visible to the naked eye.
[0364]For the determination of the said maximum concentration, the tensioning agent is gradually added to the water with deflocculating stirring at a temperature ranging from 25° C. to 50° C., and the mixture is then stirred for one hour. The mixture thus prepared is then examined after 24 hours to see if it is of uniform appearance (absence of aggregates visible to the naked eye).
[0365]The tensioning effect may be characterized by an in vitro shrinkage test.
[0366]A homogeneous mixture of the tensioning agent in water, at a concentration of 7% by weight or at the maximum concentration defined above, is prepared beforehand and as described previously.
[0367]30 μl of the homogeneous mixture are placed on a rectangular sample (10×40 mm, thus having an initial width L0 of 10 mm) of elastomer with a modulus of 20 MPa and a thickness of 100 μm.
[0368]After drying for 3 hours at 22±3° C. and 40±10% relative humidity RH, the elastomer sample has a shrunken width, noted L3h, due to the tension exerted by the applied tensioning agent.
[0369]The tensioning effect (TE) of the said polymer is then quantified in the following manner:
[0000]
‘TE’=(L0−L3h/L0)×100 as %
[0000]with L0=initial width 10 mm and
L3h=width after 3 hours of drying
[0370]The tensioning agent may be chosen from:
[0000]plant or animal proteins and hydrolysates thereof;
polysaccharides of natural origin;
mixed silicates;
colloidal particles of mineral fillers;
synthetic polymers;
and mixtures thereof.
[0371]A person skilled in the art will know how to choose, from the chemical categories listed above, the materials corresponding to the tensioning test as described below.
[0372]Mention may be made especially of:
[0000](a) plant proteins and protein hydrolysates, in particular of corn, rye, wheat, buckwheat, sesame, spelt, pea, bean, lentil, soybean and lupin,
(b) polysaccharides of natural origin, especially (a) polyholosides, for example (i) in the form of starch derived especially from rice, corn, potato, cassaya, pea, wheat, oat, etc. or (ii) in the form of carrageenans, alginates, agars, gellans, cellulose polymers and pectins, advantageously as an aqueous dispersion of gel microparticles, and (b) latices consisting of shellac resin, sandarac gum, dammar resins, elemi gums, copal resins, cellulose derivatives, and mixtures thereof,
(c) mixed silicates, especially phyllosilicates and in particular Laponites,
(d) colloidal particles of mineral fillers with a number-average diameter of between 0.1 and 100 nm and preferably between 3 and 30 nm, and chosen, for example, from: silica, silica-alumina composites, cerium oxide, zirconium oxide, alumina, calcium carbonate, barium sulfate, calcium sulfate, zinc oxide and titanium dioxide. As silica-alumina composite colloidal particles that may be used in the compositions according to the invention, examples that may be mentioned include those sold by the company Grace under the names Ludox AM, Ludox AM-X 6021, Ludox HSA and Ludox TMA,
(e) synthetic polymers, such as polyurethane latices or acrylic-silicone latices, in particular those described in patent application EP-1 038 519, such as a polydimethylsiloxane grafted with propylthio(polymethyl acrylate), propylthio(polymethyl methacrylate) and propylthio(polymethacrylic acid), or alternatively a polydimethylsiloxane grafted with propylthio(polyiso-butyl methacrylate) and propylthio(polymethacrylic acid).
[0373]Such grafted silicone polymers are especially sold by the company 3M under the trade names VS 80, VS 70 and LO21.
[0374]The tensioning agent will be present in the composition in an amount that is effective for obtaining the desired biological effect according to the invention.
[0375]By way of example, the tensioning agent may be included in the composition according to the invention in a content ranging from 0.01% to 30% by weight of active material and preferably from 1% to 30% by weight of active material relative to the total weight of the composition.
[0376]The term “active material” is intended to exclude the medium in which the tensioning agent may be dissolved or dispersed in its commercial form, for example in the case of dispersions of colloidal particles.
[0377]It is also possible, especially in order to complement and/or potentiate the effect of tensioning agents, to use agents that increase the expression of mechanoreceptors, such as agents that increase the expression of integrins.
[0378]An example that may be mentioned is an extract of rye seed, such as the product sold by Silab under the name Coheliss®.
16. Fat-Restructuring Agents
[0379]According to the invention, the term “fat-restructuring agents” means agents capable of stimulating lipogenesis and of promoting adipocyte differentiation, thus making it possible to prevent or slow down the wasting of fat contained in the skin-supporting tissues, also known as “wasting of skin fat”.
[0380]The term “skin fat” means the network of fat cells that forms the volumes on which the facial skin rests and is moulded.
[0381]These agents are intended:
[0382]to reduce the loss of skin density and/or the wasting of skin fat, in particular on the cheeks and around the eyes, and/or
[0383]to prevent the collapse and/or hollowing of the facial volumes, the loss of consistency of the skin and/or its maintenance, in particular on the cheeks and around the eyes, and/or
[0384]to improve the underlying volumes of the skin of the face and/or the neck, in particular on the cheeks, the oval of the face and around the eyes, and/or
[0385]to improve the density, springiness and maintenance of the skin, in particular on the cheeks, the oval of the face and around the eyes, and/or
[0386]to remodel the facial features, in particular the oval of the face.
[0387]Examples of fat-restructuring agents that may especially be mentioned include an extract of black tea, such as the extract of fermented black tea sold by Sederma under the name Kombuchka®, and an extract of Artemisia abrotanum, such as the product sold by Silab under the name Pulpactyl®.
17. Slimming Agents
[0388]Slimming (lipolytic) agents that may especially be mentioned include caffeine, theophylline and its derivatives, theobromine, sericosine, asiatic acid, acefylline, aminophylline, chloroethyltheophylline, diprofylline, diniprophylline, etamiphylline and its derivatives, etofylline and proxyphylline; extracts of tea, of coffee, of guarana, of mate, of cola (Cola nitida) and especially the dry extract of guarana fruit (Paulina sorbilis) containing 8% to 10% caffeine; extracts of climbing ivy (Hedera helix), of arnica (Arnica montana L), of rosemary (Rosmarinus officinalis N), of marigold (Calendula officinalis), of sage (Salvia officinalis L), of ginseng (Panax ginseng), of St.-John's wort (Hypericum perforatum), of butcher's-broom (Ruscus aculeatus L), of meadowsweet (Filipendula ulmaria L), of orthosiphon (Orthosiphon stamincus Benth), of birch (Betula alba), of pumpwood and of argan tree, extracts of ginkgo biloba, extracts of horsetail, extracts of escin, extracts of cangzhu, extracts of Chrysanthellum indicum, extracts of diosgenin-rich Dioscorea plants or pure diosgenin or hecogenin and derivatives thereof, extracts of Ballota, extracts of Guioa, of Davallia, of Terminalia, of Barringtonia, of Trema or of Antirobia, the extract of bitter orange pips; an extract of husks of cocoa beans (Theobroma cacao) such as the product sold by Solabia under the name Caobromine®.
18. Agents for Promoting the Cutaneous Microcirculation
[0389]The active agent acting on the cutaneous micro-circulation may be used for preventing dulling of the complexion and/or to improve the appearance of the area around the eyes, in particular to reduce the shadows around the eyes. It may be chosen, for example, from an extract of maritime pine bark, for instance Pycnogenol® from Biolandes, manganese gluconate (Givobio GMn® from SEPPIC), an extract of Ammi visnaga such as Visnadine from Indena, extract of lupin (Eclaline® from Silab), the protein coupling of hydrolysed wheat/palmitic acid with palmitic acid, such as Epaline 100 from Laboratoires Carilene, the extract of bitter orange blossom (Remoduline® from Silab), vitamin P and derivatives thereof, for instance methyl-4 esculetol sodium monoethanoate sold under the name Permethol® by the company Sephytal, extracts of Ruscus, of common horse chestnut, of ivy, of ginseng and of melilot, caffeine, nicotinate and derivatives thereof, lysine and derivatives thereof, for instance Asparlyne® from Solabia, an extract of black tea such as Kombuchka from Sederma; rutin salts; an extract of the alga Corallina officinalis, such as the product sold by Codif; and mixtures thereof.
[0390]As preferred agents for promoting the cutaneous microcirculation, menticn will be made of caffeine, an extract of bitter orange blossom, an extract of black tea, rutin salts and an extract of the alga Corallina officinalis.
19. Calmatives or Anti-Irritants
[0391]The term “calmative” means a compound that can reduce the sensation of stinging, itching or tautness of the skin.
[0392]As calmatives that may be used in the composition according to the invention, mention may be made of: procyannidol oligomers, vitamins E, B5 and B3, caffeine and derivatives thereof, pentacylic triterpenes and plant extracts containing them, β-glycyrrhetinic acid and salts or derivatives thereof (stearyl glycyrrhetate, 3-stearoyloxyglycyrrhetic acid or glycyrrhetinic acid monoglucuronide) and also plants containing them (e.g.: Glycyrrhiza glabra), oleanolic acid and salts thereof, ursolic acid and salts thereof, boswellic acid and salts thereof, betulinic acid and salts thereof, an extract of Paeonia suffruticosa and/or lactiflora, an extract of Laminaria saccharina, extracts of Centella asiatica, Canola oil, bisabolol, the phosphoric diester of vitamin E and C, for instance Sepivital EPC® from SEPPIC, camomile extracts, allantoin, omega-3 unsaturated oils such as musk rose oil, blackcurrant oil, Ecchium oil, fish oil or beauty-leaf oil, plankton extracts, capryloyl glycine, a mixture of water lily blossom extract and of palmitoylproline, such as the product sold under the name Seppicalm VG® by the company SEPPIC, an extract of Boswellia serrata, an extract of Centipeda cunninghami, such as the product sold under the name Cehami PF by the company TRI-K Industries, an extract of sunflower seeds, in particular Hélioxine® from Silab, an extract of Linum usitatissimum seeds, for instance Sensiline® from Silab, tocotrienols, piperonal, an extract of Epilobium angustifolium, such as the product sold under the name Canadian Willowherb Extract by the company Fytokem Products, Aloe vera, phytosterols, cornflower water, rose water, an extract of mint, in particular of mint leaves, for instance Calmiskin® from Silab, aniseed derivatives, filamentous bacteria, for instance Vitreoscilla filiformis as described in patent EP 761 204 and sold by Chimex under the name Mexoryl SBG®, an extract of rose petals, for instance Rose Flower Herbasol® extract from the company Cosmetochem, shea butter, a mixture of the waxy fraction of barley seeds obtained by supercritical CO2, of shea butter and of argan oil, for instance Stimu-tex AS® from Pentapharm, alkaline-earth metal salts, especially of strontium, a fermented extract of Alteromonas sold under the name Abyssine® by the company Atrium Biotechnologies; spring water from the Vichy basin, such as waters originating from the Célestin, Chomel, Grande-Grille, Hôpital, Lucas and Parc sources, and preferably water from the Lucas source; an extract of Eperua falcata bark, such as the product sold by the company Cognis under the name Eperuline®; an extract of Paeonia suffruticosa root, such as the product sold by the company Ichimaru Pharcos under the name Botanpi Liquid B®; and mixtures thereof.
[0393]As preferred calmatives according to the invention, use will be made of:
[0000]β-glycyrrhetinic acid and salts or derivatives thereof (stearyl glycyrrhetate, 3-stearoyloxyglycyrrhetic acid or glycyrrhetinic acid monoglucuronide) and also plants containing them (e.g. Glycyrrhiza glabra); ursolic acid and salts thereof; extracts of Centella asiatica, Canola oil, bisabolol; camomile extracts, allantoin; a mixture of extract of water lily blossom and of palmitoylproline, such as the product sold under the name Seppicalm VG® by the company SEPPIC; Aloe vera, rose water, extract of mint, in particular of mint leaves, such as Calmiskin® from Silab, filamentous bacteria such as Vitreoscilla filiformis as described in patent EP 761 204 and sold by Chimex under the name Mexoryl SBG®, an extract of rose petals such as Rose Flower Herbasol® extract from the company Cosmetochem, shea butter, a fermented extract of Alteromonas sold under the name Abyssine® by the company Atrium Biotechnologies; spring water from the Vichy basin, such as waters originating from the Celestin, Chomel, Grande-Grille, Hôpital, Lucas and Parc sources, and preferably water from the Lucas source; an extract of Eperua falcata bark, such as the product sold by the company Cognis under the name Eperuline®; an extract of Paeonia suffruticosa root, such as the product sold by the company Ichimaru Pharcos under the name Botanpi Liquid B®; and mixtures thereof.
20. Sebo-Regulating or Anti-Seborrhoeic Agents
[0394]The term “sebo-regulating or anti-seborrhoeic agents” especially means agents capable of regulating the activity of the sebaceous glands.
[0395]Mention may be made especially of:
[0396]retinoic acid, benzoyl peroxide, sulfur, vitamin B6 (or pyridoxine), selenium chloride and sea fennel;
[0397]mixtures of extract of cinnamon, of tea and of octanoylglycine such as Sepicontrol A5 TEA® from SEPPIC;
[0398]the mixture of cinnamon, sarcosine and octanoylglycine sold especially by the company SEPPIC under the trade name Sepicontrol A5®;
[0399]zinc salts such as zinc gluconate, zinc pyrrolidonecarboxylate (or zinc pidolate), zinc lactate, zinc aspartate, zinc carboxylate, zinc salicylate and zinc cysteate;
[0400]copper derivatives and in particular copper pidolate such as Cuivridone® from Solabia;
[0401]extracts of plants of the species Arnica montana, Cinchona succirubra, Eugenia caryophyllata, Humulus lupulus, Hypericum perforatum, Mentha piperita, Rosmarinus officinalis, Salvia officinalis and Thymus vulgaris, all sold, for example, by the company Maruzen;
[0402]extracts of meadowsweet (Spiraea ulmaria), such as the product sold under the name Sebonormine® by the company Silab;
[0403]extracts of the alga Laminaria saccharina, such as the product sold under the name Phlorogine® by the company Biotechmarine;
[0404]mixtures of extracts of salad burnet root (Sanguisorba officinalis/Poterium officinale), of ginger rhizomes (Zingiber officinalis) and of cinnamon bark (Cinnamomum cassia), such as the product sold under the name Sebustop® by the company Solabia;
[0405]linseed extracts, such as the product sold under the name Linumine® by the company Lucas Meyer;
[0406]Phellodendron extracts, such as those sold under the name Phellodendron extract BG by the company Maruzen or Oubaku liquid B by the company Ichimaru Pharcos;
[0407]mixtures of argan oil, of Serenoa serrulata (saw palmetto) extract and of sesame seed extract, such as the product sold under the name Regu SEB by the company Pentapharm;
[0408]mixtures of extracts of willowherb, of Terminalia chebula, of nasturtium and of bioavailable zinc (microalgae), such as the product sold under the name Seborilys® by the company Green Tech;
[0409]extracts of Pygeum afrianum, such as the product sold under the name Pygeum afrianum sterolic lipid extract by the company Euromed;
[0410]extracts of Serenoa serrulata, such as the products sold under the name Viapure Sabal by the company Actives International or those sold by the company Euromed;
[0411]mixtures of extracts of plantain, of Berberis aquifolium and of sodium salicylate, such as the product sold under the name Seboclear® by the company Rahn;
[0412]clove extract, such as the product sold under the name Clove extract powder by the company Maruzen;
[0413]argan oil, such as the product sold under the name Lipofructyl® by Laboratoires Sérobiologiques;
[0414]lactic protein filtrates, such as the product sold under the name Normaseb® by the company Sederma;
[0415]extracts of the alga Laminaria, such as the product sold under the name Laminarghane® by the company Biotechmarine;
[0416]oligosaccharides of the alga Laminaria digitata, such as the product sold under the name Phycosaccharide AC by the company Codif;
[0417]sugar cane extracts, such as the product sold under the name Policosonol® by the company Sabinsa;
[0418]sulfonated shale oil, such as the product sold under the name Ichthyol Pale® by the company Ichthyol;
[0419]European meadowsweet (Spiraea ulmaria) extracts, such as the product sold under the name Cytobiol® Ulmaire by the company Libiol;
[0420]sebacic acid, especially sold in the form of a sodium polyacrylate gel under the name Sebosoft® by the company Sederma;
[0421]glucomannans extracted from konjac tuber and modified with alkylsulfonate chains, such as the product sold under the name Biopol Beta by the company Arch Chemical;
[0422]extracts of Sophora angustifolia, such as those sold under the name Sophora powder or Sophora extract by the company Bioland;
[0423]extracts of Cinchona succirubra bark, such as the product sold under the name Red Bark HS by the company Alban Muller;
[0424]extracts of Quillaja saponaria, such as the product sold under the name Panama wood HS by the company Alban Muller;
[0425]glycine grafted onto an undecylenic chain, such as the product sold under the name Lipacide UG OR by the company SEPPIC;
[0426]the mixture of oleanolic acid and of nordihydroguaiaretic acid, such as the product sold in the form of a gel under the name AC.Net by the company Sederma;
[0427]phthalimidoperoxyhexanoic acid;
[0428]tri(C12-C13)alkyl citrate sold under the name Cosmacol® ECI by the company Sasol; tri(C14-C15)alkyl citrate sold under the name Cosmacol® ECL by the company Sasol;
[0429]10-hydroxydecanoic acid, and especially mixtures of 10-hydroxydecanoic acid, of sebacic acid and of 1,10-decanediol, such as the product sold under the name Acnacidol BG by the company Vincience; and
[0431]Preferred anti-seborrhoeic active agents that may be mentioned include:
[0432]benzoyl peroxide and vitamin B6 (or pyridoxine),
[0433]zinc salts such as zinc gluconate, zinc pyrrolidonecarboxylate (or zinc pidolate), zinc lactate, zinc aspartate, zinc carboxylate, zinc salicylate and zinc cysteate;
[0434]meadowsweet (Spiraea ulmaria) extracts, such as the product sold under the name Sebonormine® by the company Silab;
[0435]extracts of the alga Laminaria saccharina, such as the product sold under the name Phlorogine® by the company Biotechmarine;
[0436]mixtures of extracts of salad burnet root (Sanguisorba officinalis/Poterium officinale), of ginger rhizomes (Zingiber officinalis) and of cinnamon bark (Cinnamomum cassia), such as the product sold under the name Sebustop® by the company Solabia;
[0437]clove extract, such as the product sold under the name Clove extract powder by the company Maruzen;
[0438]lactic protein filtrates, such as the product sold under the name Normaseb® by the company Sederma;
[0439]European meadowsweet (Spiraea ulmaria) extracts, such as the product sold under the name Cytobiol® Ulmaire by the company Libiol;
[0440]sebacic acid, especially sold in the form of a sodium polyacrylate gel under the name Sebosoft® by the company Sederma;
[0441]glycine grafted onto an undecylenic chain, such as the product sold under the name Lipacide UG OR by the company SEPPIC;
[0442]tri(C12-C13)alkyl citrate sold under the name Cosmacol® ECI by the company Sasol; tri(C14-C15)alkyl citrate sold under the name Cosmacol® ECL by the company Sasol;
[0443]10-hydroxydecanoic acid, and especially mixtures of 1-hydroxydecanoic acid, of sebacic acid and of 1,10-decanediol, such as the product sold under the name Acnacidol BG by the company Vincience; and
[0445]Preferentially, the anti-seborrhoeic active agent is chosen from:
[0446]zinc salts such as zinc gluconate, zinc pyrrolidonecarboxylate (or zinc pidolate), zinc lactate, zinc aspartate, zinc carboxylate, zinc salicylate and zinc cysteate; and preferably zinc pyrrolidonecarboxylate (or zinc pidolate) or zinc salicylate;
[0447]clove extract, such as the product sold under the name Clove extract powder by the company Maruzen;
[0448]glycine grafted onto an undecylenic chain, such as the product sold under the name Lipacide UG OR by the company SEPPIC;
[0449]tri(C12-C13)alkyl citrate sold under the name Cosmacol® ECI by the company Sasol; tri(C14-C15)alkyl citrate sold under the name Cosmacol® ECL by the company Sasol;
[0450]and mixtures thereof.
[0451]The anti-seborrhoeic active agent is, for example, present in a content ranging from 0.1% to 10% by weight, preferably from 0.1% to 5% by weight and preferentially from 0.5% to 3% by weight relative to the total weight of the composition.
21. Astringents
[0452]According to the invention, the term “astringents” means agents for combating the dilation of the sebaceous follicles.
[0453]As astringents that may be used in the composition according to the invention, mention may be made of extracts of mushroom pulp (Polyporus officinalis), for instance Laricyl LS8865® from Cognis, extracts of Terminalia catappa and Sambucus nigra, for instance Phytofirm LS9120® from Cognis, extracts of gall nut, for instance Tanlex VE® from Ichimaru Pharcos, aluminium hydroxychloride, centella extracts (e.g. Plantactiv centella from Cognis), dicetyl dimethylammonium chloride, for instance Varisoft 432 CG® from Degussa, common horsechestnut extracts, mallow extracts, witch-hazel extracts, sweet almond extracts, marshmallow root extracts and linseed extracts, for instance Almondermin LS 3380® from Cognis, burdock extracts, nettle extracts, birch extracts, horsetail extracts, camomile extracts, for instance those sold under the name Extrapone 9 Special® by the company Symrise, skullcap extracts, European meadowsweet extracts (for example Cytobiol Ulmaire from Libiol), a mixture of extracts of white ginger, of horsetail, of nettle, of rosemary and of yucca, for instance Herb extract B1348® from Bell Flavors & Fragrances, extracts of acacia, of elm, of white willow, of cinnamon, of birch and of meadowsweet, Panama sapogenins, zinc phenolsulfonate from Interchemical, extracts of gentian, of cucumber and of walnut, the mixture of extracts of Ratanhia, of grapefruit, of gumweed and of oak gall, for instance Epilami from Alban Muller.
[0454]As preferred astringents according to the invention, use will be made of skullcap extracts, European meadowsweet extracts, meadowsweet extracts, gentian extracts and burdock extracts, and mixtures thereof.
22. Cicatrizing Agents
[0455]Examples of cicatrizing agents that may especially be mentioned include:
[0000]allantoin, urea, certain amino acids, for instance hydroxyproline, arginine, and serine, and also extracts of white lily (for instance Phytélène Lys 37EG 16295 from Indena), a yeast extract, for instance the cicatrizing agent LS LO/7225B from Laboratoires Sérobiologiques), tamanu oil, extract of Saccharomyces cerevisiae, for instance Biodynes® TRF® from Arch Chemical, oat extracts, chitosan and derivatives, for instance chitosan glutamate, carrot extracts, artemia extract, for instance GP4G® from Vincience, sodium acexamate, lavandin extracts, propolis extracts, ximeninic acid and salts thereof, rose hip oil, marigold extracts, for instance Souci Ami® Liposolible from Alban Muller, horsetail extracts, lemon peel extracts, for instance Herbasol® citron from Cosmetochem, helichrysum extracts, common yarrow extracts and folic acid.
[0456]As preferred cicatrizing agents according to the invention, use will be made of arginine, serine, folic acid, tamanu oil, sodium acexamate, horsetail extracts and helichrysum extracts, and mixtures thereof.
23. Anti-Inflammatory Agents
[0457]As particular anti-inflammatory agents that may be used according to the invention, mention may be made of cortisone, hydrocortisone, indomethacin, betamethasone, azelaic acid, acetaminophen, diclofenac, clobetasol propionate, folic acid; an extract of Eperua falcata bark, such as the product sold by the company Cognis under the name Eperuline®; an extract of Paeonia suffruticosa root, such as the product sold by the company Ichimaru Pharcos under the name Botanpi Liquid B®; and mixtures thereof.
[0458]Preferred anti-inflammatory agents that will be mentioned are azelaic acid, folic acid, an extract of Eperua falcata bark, such as the product sold by the company Cognis under the name Eperuline®; an extract of Paeonia suffruticosa root, such as the product sold by the company Ichimaru Pharcos under the name Botanpi Liquid B®; and mixtures thereof.
24. Anti-Acne Agents
[0459]In one advantageous aspect of the invention, the composition may also comprise at least one anti-acne active agent.
[0460]The term “anti-acne active agent” especially means any active agent that has effects on the specific flora of greasy skin, for instance Propionibacterium acnes (P. acnes). These effects may be bactericidal.
[0461]Antibactericidal active agents that may especially be mentioned include:
[0462]active agents and preserving agents with antimicrobial activity mentioned in patent application DE 103 24 567, which is incorporated into the present invention by reference,
[0464]the monoethanolamine salt of 1-hydroxy-4-methyl 6-trimethylpentyl-2-pyridone (INCI name: piroctone olamine), sold especially under the name Octopirox® by the company Clariant;
[0465]citronellic acid, perillic acid (or 4-isopropenylcyclohex-1-enecarboxylic acid),
[0466]glyceryl 2-ethylhexyl ether (INCI name: ethylhexylglycerine), for example sold under the name Sensiva SC 50® by the company Shulke & Mayr,
[0467]glyceryl caprylate/caprate, for example sold under the name Capmul MCM® by the company Abitec;
[0468]sodium calcium phosphosilicate, especially sold under the names Bioactive Glasspowder and Actysse Premier BG® by the company Schott Glass;
[0469]silver-based particles, for example those sold under the name Metashine ME 2025 PS by the company Nippon Sheet Glass;
[0470]hop cone extract (Humulus lupulus) obtained by supercritical CO2 extraction, such as the product sold under the name HOP CO2-TO Extract® by the company Flavex Naturextrakte,
[0471]St.-John's Wort extract obtained by supercritical CO2 extraction, such as the product sold under the name St.-John's Wort CO2-TO extract® by the company Flavex Naturextrakte,
[0472]the mixture of extracts of roots of Scutellaria baicalensis, of Paeonia suffruticosa and Glycyrrhiza glabra, such as the product sold under the name BMB-CF® by the company Naturogin,
[0473]argan tree extract, for instance Argapure LS9710® from Cognis;
[0474]bearberry leaf extracts, for instance the product sold under the name Melfade-J by the company Pentapharm;
[0475]10-hydroxy-2-decanoic acid such as Acnacidol P® from Vincience, sodium ursolate, azelaic acid, diiodomethyl p-tolyl sulfone such as Amical Flowable® from Angus, malachite powder, zinc oxide such as Zincare® from Elementis GMBH, octadecenedioic acid such as Arlatone dioic DCA® from Uniqema; ellagic acid; 2,4,4′-trichloro-2′-hydroxydiphenyl ether (or triclosan), 1-(3′,4′-dichlorophenyl)-3-(4′-chloro-phenyl)urea (or triclocarban), 3,4,4′-trichloro-carbanilide, 3′,4′,5′-trichlorosalicylanilide, phenoxy-ethanol, phenoxypropanol, phenoxyisopropanol, hexamidine isethionate, metronidazole and salts thereof, miconazole and salts thereof, itraconazole, terconazole, econazole, ketoconazole, saperconazole, fluconazole, clotrimazole, butoconazole, oxiconazole, sulfaconazole, sulconazole, terbinafine, ciclopirox, ciclopiroxolamine, undecylenic acid and salts thereof, benzoyl peroxide, 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, phytic acid, N-acetyl-L-cysteine, lipoic acid, azelaic acid and salts thereof, arachidonic acid, resorcinol, 3,4,4′-trichloro-carbanalide, octoxyglycerine or octoglycerine, octanoylglycine such as Lipacid C8GC® from SEPPIC, caprylyl glycol, 10-hydroxy-2-decanoic acid, dichlorophenylimidazoldioxolane and derivatives thereof described in patent application WO 93/18743, iodopropynyl butylcarbamate, 3,7,11-trimethyldodeca-2,5,10-trienol or farnesol, phytosphingosines; quaternary ammonium salts, for instance cetyltrimethylammonium salts and cetylpyridinium salts, and
[0477]Mention may also be made of certain surfactants with an antimicrobial effect, for instance sodium cocoamphoacetate or disodium diacetate such as Miranol C2M Conc. NP, betaines, for instance the cocoyl betaine Genagen KB from Clariant, sodium lauryl ether sulfate, for instance Emal 270 D from Kao, decyl glucoside, for instance Plantacare 2000 UP, branched C12-13 dialkyl malates, for instance Cosmacol EMI, propylene glycol monoesters, for instance propylene glycol monolaurate, monocaprylate or monocaprate, lauryldimethylamine betaine, for instance Empigen BB/LS, and also polyquaternary ammoniums such as Quaternium-24 or Bardac 2050 from Lonza and those described in patent FR 0 108 283, and mixtures thereof.
[0478]As preferred antimicrobial agents, an agent chosen from octoglycerine or octoxyglycerine, and 10-hydroxy-2-decanoic acid, and mixtures thereof, will be used in the compositions of the invention.
[0479]Other additional anti-acne active agents may be added to the abovementioned anti-acne active agents.
[0480]Mention may be made especially of active agents with bacterial anti-adhesion effects or agents that act on the biofilm of bacteria to prevent them from multiplying.
[0481]As agents for preventing and/or reducing the adhesion of microorganisms, mention may be made especially of: phytanetriol and derivatives thereof as described in patent application EP 1 529 523, plant oils such as wheatgerm oil, calendula oil, castor oil, olive oil, avocado oil, sweet almond oil, groundnut oil, jojoba oil, sesame seed oil, apricot kernel oil, sunflower oil and macadamia oil, described in patent EP 1 133 979, or certain surfactants such as disodium cocoamphodiacetate, oxyethylenated (7 EO) glyceryl cocoate, 18-hexadecenyl succinate, octoxyglyceryl palmitate, octoxyglyceryl behenate, dioctyl adipate, PPG-15 stearyl ether, and the branched C12-C13 dialkyl tartrates described in patent EP 1 129 694, and mixtures thereof.
[0482]In particular with regard to the propagation of P. acnes, or as active agents that act on the biofilm of bacteria to prevent them from proliferating, mention may be made of pentylene glycol, Nylon-66 (polyamide 66 fibres), rice bran oil, polyvinyl alcohol such as Celvol 540 PV alcohol from Celanese Chemical, rapeseed oil such as Akorex L® from Karlshamns, and fructose derivatives, and mixtures thereof.
[0483]The anti-acne active agent may be present in a content ranging from 0.01% to 10% by weight and preferably from 0.05% to 5% by weight relative to the total weight of the composition.
[0484]As a function of the nature and/or solubility of the abovementioned active agents, a person skilled in the art will know how to select the most suitable embodiment according to the invention.
[0485]The cosmetic and/or dermatological active agents will be present in one of the compositions according to the invention in a content ranging from 0.001% to 20% by weight relative to the total weight of the composition, preferably from 0.01% to 10%, even more preferentially from 0.5% to 5% and more preferably from 0.1% to 1% by weight relative to the total weight of the composition.
[0486]For “scrubbing” applications, the contents of cosmetic and/or dermatological active agents may range from 1% to 50% by weight relative to the total weight of the composition and preferably from 1% to 30% by weight relative to the total weight of the composition.
[0487]Scrubbing is a well-known means for improving the appearance and/or texture of the skin and/or the scalp, especially for improving the radiance and homogeneity of the complexion and/or for reducing the visible and/or tactile irregularities of the skin, and in particular for improving the surface appearance of the skin, for attenuating actinic lentigo, acne or chicken pox marks, and also for preventing, attenuating or combating the signs of ageing of the skin, and especially for smoothing out irregularities in the texture of the skin, such as wrinkles and fine lines.
[0488]It has the effect of removing a surface part of the skin to be treated (epidermis and possibly the upper layer of the dermis), via chemical methods.
[0489]Concrete, but in no way limiting, examples illustrating the invention will now be given.
EXAMPLE 1
Process for Obtaining a Dye from Dehydroascorbic Acid and at Least One Amino Acid
[0490]A solution containing 5% DHAA and 5% glycine (i.e. 0.5 g in 10 ml of each of the ingredients) is prepared and then heated at 60° C. for about 15 minutes. A strong red colour is observed, and the reaction is stopped by rapid cooling (crushed ice). The resulting solution is then freeze-dried to recover the dye obtained in a dry form.
COMPARATIVE EXAMPLES 2 TO 7
[0491]Formulations 2 to 7 below were prepared and stability tested for 3 months at room temperature and at 45° C.
[0000] |
| A | PEG/PPG-18/18 | 10 | 10 | 10 | 10 | 10 | 10 |
| dimethicone Dow |
| Corning 5225C |
| Formulation A = id |
| Cyclopentasiloxane | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| C | DHA | 5 | 5 | 5 | 5 | 5 | 5 |
| Deionized water | 10 | 10 | 10 | 10 | 10 | 10 |
| D | Denatured 96° | 5 | 5 | 5 | 5 | 5 | 5 |
| ethyl alcohol |
| B1 | Orange 4 | — | 0.1 | — | — | — | — |
| Red 28 | — | — | 0.1 | — | — | — |
| Green 5 | — | — | — | 0.1 | — | — |
| Red 33 | — | — | — | — | 0.1 | — |
| Dye of Example 1 | — | — | — | — | — | 0.1 |
| B | Dipropylene glycol | 10 | 10 | 10 | 10 | 10 | 10 |
| Propylene glycol | 14 | 14 | 14 | 14 | 14 | 14 |
| Butylene glycol | 9.5 | 9.5 | 9.5 | 9.5 | 9.5 | 9.5 |
| NaCl | 2 | 2 | 2 | 2 | 2 | 2 |
| Deionized water | 22 | 21.9 | 21.9 | 21.9 | 21.9 | 21.9 |
|
| *outside the invention |
[0492]The colour variations obtained with each of these compositions during storage at room temperature and at 45° C. are observed with the naked eye.
[0493]To do this, the formulations are spread onto the glossy white area of a contrast plate at a wet thickness of about 300 μm. The plates are
[0494]The results are as follows:
[0000] |
| | | | | | 7 |
| Composition | 2 | 3 | 4 | 5 | 6 | (invention) |
| ΔE |
|
| Observation of a | No | Yes | Yes | Yes | Yes | No |
| colour change at room |
| temperature |
| Observation of a | No | Yes | Yes | Yes | Yes | No |
| noticeable colour |
| change at 45° C. |
|
[0495]These results show that standard direct dyes are not compatible in formulation with DHA, in contrast with the dye according to the invention of Example 1.
EXAMPLE 8
Oil/Water Emulsion
Phase A
[0000] | Glyceryl stearate (and) PEG-100 stearate: | 2.00 g |
| Dimyristyl tartrate (and) cetearyl alcohol | 1.50 g |
| (and) C12-C15 Pareth-7 (and) PPG-25 Laureth- |
| 25: |
| Cyclohexasiloxane: | 10.00 g |
| Stearyl alcohol: | 1.00 g |
| |
Phase B
[0000] | Water: | 78.75 g |
| Phenoxyethanol: | 1.00 g |
| Pentasodium ethylenediaminetetramethylene- | 0.05 g |
| phosphate: |
| Ammonium polyacryldimethyltauramide: | 0.40 g |
| Xanthan gum: | 0.20 g |
| |
Phase C
[0000] | DHA: | 2.50 g |
| Dye obtained according to Example 1: | 2.50 g |
| |
Procedure:
[0499]Phase B is heated to about 75° C. and the ammonium polyacryldimethyltauramide is incorporated therein; the mixture is stirred until a homogeneous gel is obtained. Phase A is heated to about 75° C. The emulsion is prepared by incorporating phase A into phase B. Phase C is incorporated at 40-45° C. and stirring is continued until the emulsion is completely cool.
EXAMPLE 9
Oil/Water Emulsion
Phase A
[0000] | Oxyethylenated polymethylcetyl dimethyl | 1.5 | g |
| methylsiloxane |
| Polyglyceryl isostearate | 0.5 | g |
| Dimethicone | 2.05 | g |
| Cyclopentasiloxane | 4 | g |
| Isononyl isononanoate | 6.66 | g |
| Preserving agent | 0.15 | g |
| |
Phase B
[0000] | Water | qs |
| Propylene glycol | 3 | g |
| Magnesium sulfate | 1.75 | g |
| Methyl paraben | 0.2 | g |
| Preserving agent | 0.3 | g |
| |
Phase C
Phase D
[0000] | DHA: | 2.50 g |
| Dye obtained according to Example 1: | 2.50 g |
| |
Procedure:
[0504]Phase A and phase B are separately homogenized at room temperature with stirring. The emulsion is prepared by incorporating phase B into phase A. Phases C and D are incorporated with stirring.














